Acropyga rubescens
| Acropyga rubescens | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Formicinae |
| Tribe: | Plagiolepidini |
| Genus: | Acropyga |
| Species group: | acutiventris |
| Species: | A. rubescens |
| Binomial name | |
| Acropyga rubescens Forel, 1894 | |
| Synonyms | |
| |
Nothing is known about the biology of this ant.
Identification
LaPolla (2004) - A member of the Acropyga acutiventris species group. Worker: 11 segmented antennae; mandible with 5 teeth and indistinct ridges that run along dorsal surface fading toward median portion of mandibles; scapes > 0.9 mm; many erect hairs on head, mesosoma and gaster giving ant a "spiky appearance." Queen: As in worker with modifications expected for caste. Male: As in Acropyga acutiventris, except hairier and penis valve differences. Compare with Acropyga acutiventris.
This species is the largest known Acropyga species, and is closely related to Acropyga acutiventris.
Keys including this Species
Distribution
Distribution based on Regional Taxon Lists
Indo-Australian Region: Singapore.
Oriental Region: India (type locality), Sri Lanka.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
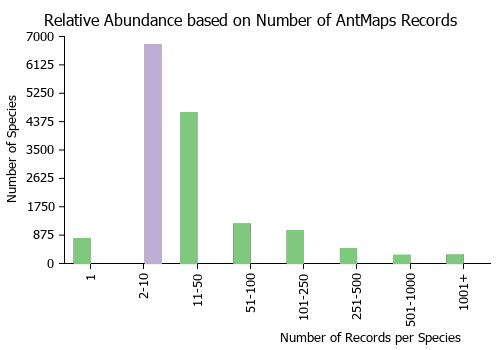
|
Biology
|
Castes
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- rubescens. Acropyga acutiventris var. rubescens Forel, 1894c: 418 (w.q.m.) INDIA (Karnataka), SRI LANKA.
- [Misspelled as pubescens by Chapman & Capco, 1951: 211.]
- Subspecies of acutiventris: Viehmeyer, 1916a: 146; Emery, 1925b: 28; Chapman & Capco, 1951: 211; Bolton, 1995b: 58.
- Status as species: LaPolla, 2004a: 33 (redescription); Bharti, Guénard, et al. 2016: 23.
- Senior synonym of bugnioni: LaPolla, 2004a: 33.
- Senior synonym of rubens: Emery, 1925b: 28; Bolton, 1995b: 58; LaPolla, 2004a: 33.
- bugnioni. Acropyga acutiventris subsp. bugnioni Forel, 1911i: 226 (w.q.) SRI LANKA.
- Subspecies of acutiventris: Emery, 1925b: 28; Chapman & Capco, 1951: 211; Bolton, 1995b: 57.
- Junior synonym of rubescens: LaPolla, 2004a: 33.
- rubens. Acropyga acutiventris subsp. rubens Forel, 1911e: 286 (w) (no state data).
- [Note: this name most probably in error for rubescens.]
- Junior synonym of rubescens: Emery, 1925b: 28; Bolton, 1995b: 58; LaPolla, 2004a: 33.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
LaPolla (2004) - Acropyga rubescens has been elevated to species status based on 6 points of distinction from A. acutiventris: 1) setation of A. rubsecens is distinct: a) much denser hairs in general found across body; b) hairs on posterior margin of head often very long, much longer than seen in A. acutiventris; c) mesosomal hairs longer and denser; 2) A. rubescens larger overall; 3) with longer scapes (higher SI); 4) dorsal mandibular striate sculpture thinner disappearing about mid-way toward mandible articulation point with head; 5) shapes of penis valves differ; 6) both "forms" are sympatric in India/Sri Lanka region.
Description
Worker
LaPolla (2004) - (n=7): TL: 3.39-5.11; HW: 0.891-1.16; HL: 0.915-1.06; SL: 0.914-1.16; ML: 1.07-1.31; GL: 1.35-2.74; CI: 91.53-109.43; SI: 97.39-106.67.
Head: brownish-yellow to reddish-yellow; head about as broad as long; posterior margin concave; covered in a thick layer of appressed hairs, with many scattered, long erect hairs especially along posterior margin; eyes relatively large, placed at lower 114 of head; 11 segmented, incrassate antennae; scape nearly reaches or surpasses posterior margin up to length of pedicel; clypeus broad, slightly convex medially with many erect hairs, the longest ones along anterior clypeal margin; mandible with 5 uneven teeth; 3rd and 5th tooth (from apical to basal) smaller than others; dorsal surface of mandible covered in many erect hairs and with indistinct ridges originating near base of teeth and fading entirely toward middle of mandible; slight gap present between inner mandibular surface and anterior clypeal margin. Mesosoma: brownish-yellow to reddish-yellow; mesosoma covered throughout in dense layer of appressed hairs with scattered erect hairs of varying lengths; pronotum in lateral view typically with short shelf before rising sharply toward mesonotum; mesonotum rounded with many long erect hairs (many times longer than shortest erect hairs); metanotal area distinct; propodeum rounded, below level of mesonotum, propodeal dorsum flat before steep declivity; propodeum with dense layer of erect and appressed hairs. Gaster: brownish-yellow to reddish-yellow, darker than head and mesosoma; petiole thick and erect reaching height of propodeum; yellow to light brownish-yellow; gaster covered in thick layer of appressed hairs with many scattered erect hairs throughout giving a "spiky" appearance.
Queen
LaPolla (2004) - (n= l): TL: 6.16; HW: 1.36; HL: 1.36; SL: 1.34; ML: 2.23; GL: 2.57; CI: 100; SI: 98.53. As in worker with modifications expected for caste.
Male
LaPolla (2004) - (n= l ): TL: 3.52; HW: 0.644; HL: 0.665; SL: 0.611 ; ML: 1.25; GL: 1.6; CI: 96.84; SI: 94.88.
Head: brownish-yellow to reddish-yellow, darker around three prominent ocelli; head about as broad as long; covered in layer of appressed hairs with suberect to erect hairs; eyes large, breaking outline of head in full frontal view; 12 segmented, slightly incrassate antennae; scape surpasses posterior margin by about length of first two funicular segments; clypeus broad, relatively flat, with scattered short erect hairs; mandible with 4 teeth; a gap exists between inner mandibular margin and anterior clypeal margin. Mesosoma: light brownish-yellow to reddish-yellow; pronotum short and collar-like; mesonotum large, rounded anteriorly; mesonotum dorsum flat, with layer of shorter appressed hairs and scattered longer erect hairs throughout; propodeum lower than mesonotum and scutellum; declivity not distinct from propodeum. Gaster: petiole thick and erect; gaster brownish-yellow to reddish-yellow, darker dorsally; covered in dense layer of appressed hairs with scattered erect hairs throughout. Genitalia: in lateral view parameres thick, tapering to a rounded apex; parameres with medial dorsolateral extensions; cuspi short, bent toward approximately middle of digiti, where they meet with short, peg-like teeth at apex; digiti long and erect with short peg-like teeth where cuspi meet, apex rounded.
Type Material
LaPolla (2004):
Acropyga acutiventris rubescens Forel, 1894: 418, (w.q.m.). 10 syntype workers, 2 syntype queens, 3 syntype males, INDIA: Kanara (Bell) (American Museum of Natural History) (The Natural History Museum) (Museum of Comparative Zoology) [examined]. The designated lectotype is a worker labeled JSL TYPE # 111 and is deposited at MCZC.
Acropyga acutiventris bugnioni Forel, 1911c: 226 (w.q.). 2 syntype workers, SRI LANKA: no specific locality given (Naturhistorisches Museum, Basel) [examined]. NEW SYNONYM.
Acropyga acutiventris rubens Forel, 19l1a: 286 (w.). Holotype worker, no locality provided (depository unknown) [not examined]. Synonym of A. rubescens by Emery, 1925 [probably a miss-spelling of rubescens (Bolton, 1995)] (here confirmed).
References
- Biinzli, G.H. 1935. Untersuchungen iiber coccidophile Ameisen aus den Kaffeefelden von Surinam. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 16:455-593.
- Brown, W.L., Jr. 1945. An unusual behavior pattern observed in a Szechuanese ant. Journal of the West China Border Research Society 15:185-186.
- Buschinger, J., J. Heinze & K. Jessen. 1987. First European record ofa queen ant carrying a mealybug during her mating flight. NatUlwissenschaften 74:139-140.
- Eberhard, W.G. 1978. Mating swarms ofa South American Acropygia [sic.] (Hymenoptera: Formicidae). Entomological News 89(1 & 2):14-16.
- Eisner, T. 1957. A comparative morphological study ofthc proventriculus of ants (Hymenoptera: Formicidae). Bulletin ofthe Museum of Comparative Zoology 116:439-490.
- Emery, C. 1925d. Hymenoptera. Fam. Formicidae. Subfam. Formicinae. Genera Insectorum 183: 1-302 (page 28, senior synonym of rubens)
- Forel, A. 1894c. Les Formicides de l'Empire des Indes et de Ceylan. Part IV. J. Bombay Nat. Hist. Soc. 8: 396-420 (page 418, worker, queen, male described)
- George, M.E., Prasad, G. 2023. First record of the genus Acropyga Roger, 1862 (Hymenoptera: Formicidae: Formicinae) in Kerala, India. Journal of Threatened Taxa 15(1): 22515–22521 (doi:10.11609/jott.2023.15.1.22355-22558).
- Holldobler B . & E.O. Wilson. 1990. The Ants. Belknap Press, Cambridge, Massachusetts, 732 pp.
- Johnson, c., D. Agosti, J.H. Delabie, K. Dumpert, OJ. Williams, M. von Tschimhaus & U. Maschwitz. 2001 . Acropyga and Azteca Ants with Scale Insects: 20 Million Years ofIntimate Symbiosis. American Museum Noviates 3335:1-18.
- LaPolla, J.S. 2004a. Acropyga of the world. Contributions of the American Entomological Institute. 33(3):1-130. (page 3, raised to species: new status)
- LaPolla, J.S., S.P. Cover & U.G. Mueller. 2002. Natural history of the mealybug-tending ant Acropyga epedana, with descriptions of the male and queen castes. Transactions of the American Entomological Society 128(3):367-376.
- Prins, AJ. 1982. Review of Anoplolepis with reference to male genitalia, and notes on Acropyga. Annals of the South African Museum 89:215-247.
- Wang, W.Y., Soh, E.J.Y., Yong, G.W.J., Wong, M.K.L., Benoit Guénard, Economo, E.P., Yamane, S. 2022. Remarkable diversity in a little red dot: a comprehensive checklist of known ant species in Singapore (Hymenoptera: Formicidae) with notes on ecology and taxonomy. Asian Myrmecology 15: e015006 (doi:10.20362/am.015006).
- Weber, N.A. 1944. The Neotropical coccid-tending ants of the genus Acropyga Roger. Annals of the Entomological Society of America 37:89-122.
- Wheeler, G.C. & J.C. Wheeler. 1953. The ant larvae of the subfamily Formicinae. Annals of the Entomological Society of America 46:126-171.
- Wheeler, W.M. 1935b. Ants of the genus Acropyga Roger, with description ofa new species. Journal of the New York Entomological Society 43:321-329.
- Williams, D J . 1998. Mealybugs of the genera Eumyrmococcus Silvestri and Xenococcus Silvestri associated with the ant genus Acropyga Roger and a review of the subfamily (Hemiptera, Coccoidea, Pseudoccidae). Bulletin of the British Museum (Natural History)(Entomology) 67:1-64.
References based on Global Ant Biodiversity Informatics
- Chapman, J. W., and Capco, S. R. 1951. Check list of the ants (Hymenoptera: Formicidae) of Asia. Monogr. Inst. Sci. Technol. Manila 1: 1-327
- Forel A. 1911. Ameisen aus Ceylon, gesammelt von Prof. K. Escherich (einige von Prof. E. Bugnion). Pp. 215-228 in: Escherich, K. Termitenleben auf Ceylon. Jena: Gustav Fischer, xxxii + 262 pp.
- LaPolla J.S. 2004. Acropyga (Hymenoptera: Formicidae) of the world. Contributions of the American Entomological Institute 33(3): 1-130.

