Ants and Bacteria
Studies have shown bacteria provide
- amino acid provisioning by Blochmannia (g-Proteo-bacteria) in Camponotus ants (Feldhaar et al., 2007)
- Cephalotes essential amino acids (Hu et al., 2018)
- Cardiocondyla with useful intermediate metabolites (Klein et al., 2016).
- Tetraponera dependent on honeydew from Pseudococcidae (scale insects): oxidative recycling of nitrogenous-rich metabolic waste by 'domesticated' root-nodule bacteria (Billen & Buschinger 2000, van Borm et al. 2002, Stoll et al. 2007)
Mollicutes
Recent studies have shown that huge numbers of bacteria called Mollicutes live in the leafcutter ants’ guts. These bacteria do not make the ants sick, so they were thought to be somehow beneficial. Now, Sapountzis et al. (2018) show that the two most common types of Mollicutes found in leafcutter ants evolved to make fungus farming more efficient. The complete genomes of two Mollicutes strains were analyzed and compared to the ones found in other insects. The results showed that both types of Mollicutes can turn excess quantities of the amino acid arginine into a nitrogen-rich fertilizer the ants deposit on their fungal gardens as feces. This helps the ants produce more food. One of the two types can also decompose citrate from plant sap and fruit juice consumed by the ants. This produces acetate, which supplements the ants’ fungal diets and provides extra energy. The unique energy-producing Mollicutes may explain why leafcutter ants evolved larger colonies and sustain higher levels of worker activity than other species of fungus-growing ants. The genome data also showed that both types of Mollicutes have costly defense systems to protect themselves against bacteria-destroying viruses. Many bacteria do not invest is such systems, but the Mollicutes may be able to afford them because their ant hosts provide them with plenty of food. This suggests that both the ants and the Mollicutes benefit from their symbiotic relationship.
Sapountzis et al. (2018) - The two most common Mollicute strains EntAcro1 and EntAcro10 (cf. Sapountzis et al., 2015) were examined in thirteen Panamanian fungus-growing ant species and compared these abundances with the typical spectrum of forage-material that different fungus-farming ants collect and use as compost to manure their fungus-gardens (Kooij et al., 2014a; Leal and Oliveira, 2000; Shik et al., 2016). The Panamanian fauna of attine ants encompasses nine of the 17 known genera, including the three most basal genera (Apterostigma, Mycocepurus and Myrmicocrypta), two other basal genera (Cyphomyrmex and Mycetophylax) being more closely related to the Trachymyrmex and Sericomyrmex lineages that arose and diversified while rearing gongylidia-bearing cultivars, and finally the Atta and Acromyrmex leaf-cutting ants who came to practice fungus-farming at an ‘industrial’ scale (Branstetter et al., 2017; Schultz and Brady, 2008; Mueller et al., 1998).
At the Panama site where we conducted our study, the EntAcro1 and EntAcro10 symbionts are the most common Entomoplasmatales strains associated with attine ants and they represent the majority of sequence reads (>40% jointly for both EntAcro symbionts that were obtained from these ants in field colonies and >50% in captive colonies fed ad libitum; Sapountzis et al., 2015). Our study thus captured much of the qualitative and quantitative biodiversity of abdominal Mollicutes endosymbionts. We show that these two symbionts are phylogenetically distant and therefore evolved independently, but that their gene contents reflect convergent adjustment to life as ant symbionts when compared to related Mesoplasma and Spiroplasma bacteria associated with other arthropods or plants (Figure 1—figure supplement 3). These convergences primarily relate to carbohydrate metabolism, consistent with patterns of bacterial adaptation being generally based on substrate utilization (Lo et al., 2015; Pa´l et al., 2005).
The loss of the arginine synthesis pathway in the basal attine ants (Nygaard et al., 2011; Suen et al., 2011) has been instrumental in making their fungus-farming symbiosis obligate (Nygaard et al., 2016). The selection regime that caused this loss remains unknown (Nygaard et al., 2016; Jesˇovnik et al., 2016), but it is reasonable to assume that outsourcing the production of this most nitrogen-rich amino acid to fungal cultivars gave complementary efficiency benefits even though it also generated symbiotic dependency. For symbiotic division of labor to be sustainable under variable environmental conditions, average levels of fungal arginine production would have to be higher than the minimally sufficient level to avoid occasional windows of fatal shortage in the symbiosis as a whole. Symbiotic dependency may thus have created a niche for Mollicutes symbionts to ensure that surplus arginine is recycled as NH3 to provide the most efficient manure for new garden growth.
The only other ant lineage in which Entomoplasmatales (Mollicutes) endosymbionts have so far been abundantly found are the army ants (Funaro et al., 2011). These ants are exclusive predators of mostly invertebrate prey (Kronauer, 2009) and 16S rDNA sequences of their Mollicutes sym- bionts suggested they are closely related to EntAcro1 but rather distantly to EntAcro10 (Funaro et al., 2011). It is intriguing that the Dacetine sister lineage of the fungus-farming ants are also specialized predators (Branstetter et al., 2017; Ward et al., 2015). It would thus be interesting to clarify whether also Dacetine ants have Entomoplasmatales symbionts, how (un)related they would be to the EntAcro symbionts of the fungus-growing ants, and whether army ants acquired their Mollicutes horizontally from preying upon on attine ants (Powell and Clark, 2004).
Nitrogen-recycling bacteria
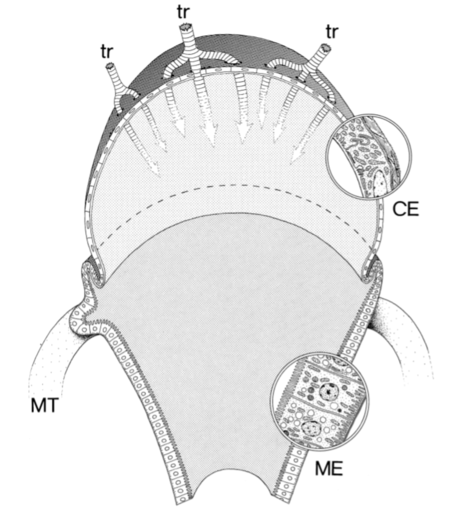
Species of the Tetraponera nigra-group in SE Asia live exclusively inside twigs or bamboo, and numerous pseudococcids (scale insects) provide the ants with honeydew (Buschinger et al. 1994). Workers are rarely seen foraging outside their nests, so the ants depend almost entirely on this amino acid-deficient honeydew diet. In Tetraponera attenuata, Tetraponera binghami and Tetraponera nitida, a unique pouch-shaped organ at the junction of the midgut and the intestine is filled with a dense mass of bacteria (Billen & Buschinger 2000). This bacterial pouch is surrounded by a network of intruding tracheae and Malpighian tubules, transporting ample oxygen and nitrogen-rich metabolic wastes to the pouch. The epithelium at the base of the pouch is specialized for absorption (Billen & Buschinger 2000), but the contorted shape of this region ensures that gut fragments cannot enter.
The bacteria cultured in the gut pouch of Tetraponera binghami are distinct from the nitrogen-recycling symbionts found in other insects (van Borm et al. 2007). Instead they are close relatives of root-associated nitrogen-fixing bacteria, and function to reintegrate metabolic nitrogen waste of the ants into the bacteria's metabolic pathways, then release amino acid precursors in the pouch where they can be absorbed by the ant’s proximal epithelium.
Comparative study of the gut microbiota in T. nigra-group, Stoll et al. 2007 Bartonella and Rhizobium (order Rhizobiales)
Related webpages
References
- Billen, J., Buschinger, A. 2000. Morphology and ultrastructure of a specialized bacterial pouch in the digestive tract of Tetraponera ants (Formicidae, Pseudomyrmecinae). Arthropod Structure & Development 29, 259-266.
- de Souza, D.J., Bézier, A., Depoix, D., Drezen, J.-M., Lenoir, A. 2009. Blochmannia endosymbionts improve colony growth and immune defence in the ant Camponotus fellah. BMC Microbiology 9, 29 (doi:10.1186/1471-2180-9-29).
- Sapountzis, P., M. Zhukova, J. Z. Shik, M. Schiott, and J. J. Boomsma. 2018. Reconstructing the functions of endosymbiotic Mollicutes in fungus-growing ants. eLife. 7:31. doi:10.7554/eLife.39209
- Stoll S, Gadau J, Gross R, Feldhaar H 2007. Bacterial microbiota associated with ants of the genus Tetraponera. Biol. J. Linn. Soc. 90: 399–412.
- Van Borm, S., Buschinger, A., Boomsma, J.J., Billen, J. 2002. Tetraponera ants have gut symbionts related to nitrogen-fixing root-nodule bacteria. Proc. R. Soc. Lond. B, 269, 2023-2027
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||