Camponotus americanus
| Camponotus americanus | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Formicinae |
| Tribe: | Camponotini |
| Genus: | Camponotus |
| Subgenus: | Camponotus |
| Species complex: | herculeanus |
| Species: | C. americanus |
| Binomial name | |
| Camponotus americanus Mayr, 1862 | |
| Synonyms | |
| |
Photo Gallery
Identification
The following information is derived from Mackay, New World Carpenter Ants (2019)
Compare with Camponotus castaneus, Camponotus herculeanus, Camponotus modoc, Camponotus novaeboracensis.
The majors, minors and females of C. americanus are relatively shiny and with a dark reddish-brown head and brown gaster and often a lighter brown mesosoma. The cheeks and malar area have a number of erect and suberect setae, pubescence is sparse and noticeable only on the dorsum and partially on the sides of the mesosoma and on the gaster. The clypeus is elevated longitudinally along the midline but does not form a carina. The head is coriaceous, or finely punctate, with the few scattered and insignificant, deeper punctures.
The male is a large, dark brown specimen that does not appear to have characteristics to separate it from other large male Camponotus.
Comparisons
Camponotus americanus can be separated from most other species in the subgenus Camponotus by the relatively long scapes and the relatively shiny surfaces. The long scapes will separate C. americanus from other species that are relatively smooth, such as Camponotus herculeanus (southern Canada and most of US) and even some of the species in the subgenus Tanaemyrmex. The shiny gaster would separate C. americanus from others that have a dull gaster with short pubescence, such as Camponotus modoc (southern Canada and most of US, northern Mexico).
Camponotus americanus can be separated from Camponotus novaeboracensis (southern Canada and most of US) as the punctures on the head are fine and insignificant and mesosoma is brown, instead of red.
It is difficult to separate C. americanus from Camponotus castaneus (SE Canada, eastern US), of which it was once considered to be a subspecies. The easiest character is the presence of numerous erect and suberect setae on the cheeks and malar area of the major, an area where erect setae are usually absent in majors of C. castaneus. Additionally, the scape of C. americanus is round at the base, whereas it is flattened and often widened in C. castaneus. It is usually necessary to have at least 1 major in a series to identify C. americanus. It is not unusual to find individual minors that lack erect setae on the malar area or have a couple of setae on one side and none on the other, making identification of series that contain only minors difficult or impossible. The two species are obviously similar and both must be placed in the same subgenus. As C. castaneus is clearly a member of Camponotus (based on the flattened clypeus, with only evidence of a raised area posteriorly), C. americanus must also be a member of the subgenus Camponotus.
Distribution
The following information is derived from Mackay, New World Carpenter Ants (2019)
Camponotus americanus is found in habitats ranging from Quercus oak forests, dense live oak, oak evergreen forest, oak-hickory-dogwood forest, honey and black oak - white oak forest, sand pine oak scrub, turkey oak, Juniperus ashei forests, Cornus florida forests, shale barrens, wet flatwoods and hardwoods, long leaf pine forests, saw palmetto, saw grass marsh, and red maple forests, often in dense forest. Hill (2012) found it in forested areas adjacent to glades. It is also found in grasslands and shrublands (Barber, 2015) as well as deciduous and mixed forest (Mac-Gown and Brown, 2006).
Davis (2009) found it in numerous habitats including swamps/bottomland hardwood forests, recently cleared land, upland pine, pine woodland/longleaf pine savanna, upland deciduous forest, mesic deciduous forest, upland mixed forest, grassland and maritime forest.
Latitudinal Distribution Pattern
Latitudinal Range: 45.47° to 24.48472222°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Nearctic Region: United States (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
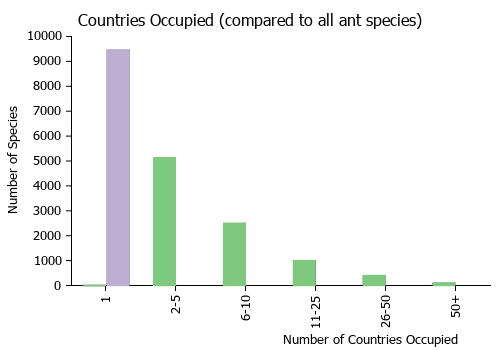
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
|
Biology
A nest chamber was found under a small downed pitch pine trunk in an open pitch pine forest (southwestern Rhode Island 41°25′59″N 71°41′19″W / 41.43292°N 71.68864°W. The chamber contained brood and pupae, and off to one side was a hole leading down into their nest. The soil was sandy. The workers initially were frantic, with some running away and others running into the hole into their nest. After a few minutes a few workers were methodically coming out of the nest, picking up brood, and bringing what they could carry into the nest. (Lubertazzi July 4, 2018, DL04664).
The following information is derived from Mackay, New World Carpenter Ants (2019)
Camponotus americanus is one of the few species of the subgenus Camponotus that normally nests in the soil (Wheeler, 1917a), without a mound or under rocks (Buren, 1944), instead of in wood. Often there is simply a hole in the ground. It rarely nests in rotten logs and may be a house pest (Hansen and Klotz, 2005; Walker, 2013). Nests are located in red or brown clay soils, dark brown sand, to rocky soils, often in the shade, and can be collected under less than 5 cm of litter. In warm sites, C. americanus tends to occupy relatively cool chambers (Diamond et al., 2012).
Nests are small (Wheeler 1905a). One nest had three dealate females. Xenodusa cava (Coleoptera Staphylinidae) overwinters in the nest (Ellison et al., 2012).
Buren (1944) reported sexuals in nests in April and May so they evidently overwinter as adults. We found sexuals in nests in April (Texas). A flight occurred at 20:00-21:00, on 22-vi-2009, air temp 25.6°, after earlier rain (Georgia). Males were collected at blacklights in June, and in malaise traps.
Camponotus americanus appears to rely on a few, highly capable foragers (Pearce-Duvet et al., 2011), which can be found foraging diurnally (09:00 - 20:00) on the soil surface and in vegetation and are attracted to peanut butter baits. Foragers were also collected in pitfall traps. Ellison et al. (2012) report it as a nocturnal omnivore, which tend aphids (Williams et al., 2011), as well as the magnolia scale Neolecanium cornuparvum (Vanek and Potter, 2010). They prey on the red oak borer Enaphalodes rufulus (Coleoptera: Cerambycidae) (Muilenburg et al., 2008).
Camponotus americanus is infected by the fungus Ophiocordyceps unilateralis sensu lato (de Bakker et al., 2014). They are also the host of the endosymbiotic bacterium Candidatus Blochmannia (Degnan et al., 2004) as well as Wolbachia bacteria (Wernegreen et al., 2009).
Eisner and Wilson (1952) discuss the morphology of the proventriculus. Hölldobler and Engel-Siegel (1984) discuss the lack of the metapleural gland in this species.
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a host for the fungus Ophiocordyceps unilateralis (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
- This species is a host for the fungus Ophiocordyceps unilateralis (a pathogen) in North America (Shrestha et al., 2017).
Flight Period
| X | X | X | |||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
Source: antkeeping.info.
- Check details at Worldwide Ant Nuptial Flights Data, AntNupTracker and AntKeeping.
- Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Life History Traits
- Queen number: monogynous (Frumhoff & Ward, 1992)
Castes
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- americanus. Camponotus americanus Mayr, 1862: 661 (w.q.) U.S.A. (Louisiana).
- Emery, 1893i: 674 (m.); Wheeler, G.C. & Wheeler, J. 1968: 216 (l.).
- Combination in C. (Camponotus): Emery, 1925b: 74.
- Junior synonym of castaneus: Mayr, 1886d: 420; Cresson, 1887: 255; Dalla Torre, 1893: 223.
- Subspecies of castaneus: Emery, 1893i: 674; Emery, 1895c: 336; Emery, 1896d: 372 (in list); Forel, 1904b: 381; Wheeler, W.M. 1905f: 402; Wheeler, W.M. 1906b: 22; Wheeler, W.M. 1910d: 323 (redescription); Wheeler, W.M. 1910g: 571; Wheeler, W.M. 1913c: 117; Wheeler, W.M. 1916m: 600; Wheeler, W.M. 1917i: 465; Emery, 1925b: 74; Smith, M.R. 1930a: 6; Wheeler, W.M. 1932a: 13; Dennis, 1938: 300; Wesson, L.G. & Wesson, R.G. 1940: 103; Buren, 1944a: 294; Smith, M.R. 1951a: 839.
- Status as species: Roger, 1863b: 5; Mayr, 1863: 398; Creighton, 1950a: 365; Eisner & Wilson, 1952: 47; Smith, M.R. 1958c: 142; Carter, 1962a: 7 (in list); Smith, M.R. 1967: 366; Smith, D.R. 1979: 1425; DuBois & LaBerge, 1988: 145; Deyrup, et al. 1989: 100; Wheeler, G.C., et al. 1994: 305; Bolton, 1995b: 85; Coovert, 2005: 163; Hansen & Klotz, 2005: 81; MacGown & Forster, 2005: 65; MacGown, et al. 2007: 10; Ellison, et al. 2012: 117; Deyrup, 2017: 186; Mackay, 2019: 163 (redescription).
- Senior synonym of rufinasis: Creighton, 1950a: 365; Smith, M.R. 1958c: 142; Smith, D.R. 1979: 1425; Bolton, 1995b: 85; Mackay, 2019: 163.
- rufinasis. Camponotus (Camponotus) castaneus st. rufinasis Santschi, 1936b: 204 (s.w.) U.S.A. (Oklahoma).
- Status as species: Santschi, 1937h: 380.
- Subspecies of castaneus: Smith, M.R. 1951a: 839.
- Junior synonym of americanus: Creighton, 1950a: 365; Smith, M.R. 1958c: 142; Smith, D.R. 1979: 1425; Bolton, 1995b: 121; Mackay, 2019: 163.
Type Material
Types not found by Mackay (2019).
Description
The following information is derived from Mackay, New World Carpenter Ants (2019)
Major worker measurements (mm): HL 3.04 - 3.50, HW 2.70 - 3.54, SL 2.96 - 3.18, EL 0.66 - 0.71, CL 1.00 - 1.16, CW 1.15 - 1.30, WL 4.12 - 4.60, FFL 2.56 - 2.92, FFW 0.74 - 0.85. Indices: CI 89 - 101, SI 91 - 97, CLI 112 - 115, FFI 29.
Mandible with 5 teeth; anterior border of clypeus straight or slightly convex, depending on view; head somewhat heart-shaped, narrowed anteriorly, with strongly concave posterior margin; eyes fail to reach sides of head by about 1 minimum diameter; scape extends 1-2 funicular segments past posterior lateral corner of head; propodeum weakly angulate between 2 faces, dorsopropodeum longer than posteropropodeum; petiole narrow as seen in profile, with convex apex, as seen from front.
Erect and suberect setae abundant, specifically on clypeus, cheeks, malar area, frontal carinae, extending back to posterior margin, dorsum of mesosoma, petiole and all surface of gaster, absent on sides of head near eyes, on posterior lateral corners, on scapes (except apex) and tibiae, except for scattered bristles on flexor surfaces.
Head predominantly punctate, posteriorly coriaceous, with few scattered insignificant larger punctures, mesosoma coriaceous, gaster very finely, transversely striolate, sides of head shining, side of mesosoma glossy and shining, dorsal surface of gaster smooth and glossy.
Head usually dark reddish brown, mesosoma usually medium brown, legs usually light brown, gaster usually medium brown.
Minor worker measurements (mm): HL 1.82 - 2.96, HW 1.48 - 2.52, SL 1.92 - 2.88, EL 0.48 - 0.61, CL 0.53 - 0.86, CW 0.78 - 1.08, WL 2.48 - 3.80, FFL 1.58 - 2.44, FFW 0.46 - 0.73. Indices: CI 81 - 85, SI 97 - 105, CLI 125 - 148, FFI 29 - 30.
Minor worker similar to major worker, except head more elongate, eyes nearly reach sides of head, scapes extend up to ½ length past posterior lateral corner of head, pilosity, sculpture and color as in major worker.
Female measurements (mm): HL 3.32 - 3.50, HW 3.26 - 3.68, SL 2.88 - 3.00, EL 0.81 - 0.86, CL 1.03 - 1.14, CW 1.21 - 1.35, WL 5.30 - 5.66, FFL 2.84 - 2.98, FFW 0.74 - 0.88. Indices: CI 98 - 105, SI 84 - 87, CLI 116 - 119, FFI 26 - 29.
Female similar to major worker; head somewhat heart-shaped, posterior margin weakly concave; eyes fail to reach sides of head by about ¼ - ½ minimum diameter; scape extends 1-2 funicular segments past posterior lateral corner of head; pilosity, sculpture and color is in major worker.
Male measurements (mm): HL 1.62 - 1.92, HW 1.44 - 1.68, SL 2.16 - 2.38, EL 0.58 - 0.66, CL 0.48 - 0.55, CW 0.73 - 0.79, WL 3.64 - 4.44, FFL 2.56 - 2.86, FFW 0.48 - 0.54. Indices: CI 88 - 89, SI 124 - 133, CLI 143 - 153, FFI 19.
Males moderately large, setae scattered over entire surface of clypeus, scape extends ½ length past posterior lateral corner of head, cheeks with erect and suberect setae, head dark brown, mesosoma, legs and gaster medium to dark brown.
Worker Morphology
 Explore: Show all Worker Morphology data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Worker Morphology data or Search these data. See also a list of all data tables or learn how data is managed.
• Caste: polymorphic
References
- Mackay, W.P. 2019. New World Carpenter Ants of the Hyperdiverse genus Camponotus. Volume 1. Introduction, keys to the subgenera and species complexes and the subgenus Camponotus: 412 pp. Lambert Academic Publishing.
- Buren, W. F. 1944a. A list of Iowa ants. Iowa State Coll. J. Sci. 18: 277-312.
- Carroll, T.M. 2011. The ants of Indiana (Hymenoptera: Formicidae). M.S. thesis, Purdue University.
- Chick, L.D., Lessard, J.-P., Dunn, R.R., Sanders, N.J. 2020. The coupled influence of thermal physiology and biotic interactions on the distribution and density of ant species along an elevational gradient. Diversity 12, 456 (doi:10.3390/d12120456).
- Creighton, W. S. 1950a. The ants of North America. Bulletin of the Museum of Comparative Zoology 104: 1-585 (page 365, Revived status as species, and senior synonym of rufinasis)
- Davis, T. 2009. The ants of South Carolina (thesis, Clemson University).
- Eisner, T.; Wilson, E. O. 1952. The morphology of the proventriculus of a formicine ant. Psyche (Camb.) 59: 47-60.
- Ellison, A. M., N. J. Gotelli, E. J. Farnsworth, and G. D. Alpert. 2012. A Field Guide to the Ants of New England. Yale University Press, New Haven.
- Emery, C. 1893k. Beiträge zur Kenntniss der nordamerikanischen Ameisenfauna. Zool. Jahrb. Abt. Syst. Geogr. Biol. Tiere 7: 633-682 (page 674, male described, Revived from synonymy as subspecies of castaneus)
- Emery, C. 1925d. Hymenoptera. Fam. Formicidae. Subfam. Formicinae. Genera Insectorum 183: 1-302 (page 74, Combination in C. (Camponotus))
- Hansen, L.D. & Klotz, J.H. 2005. Carpenter ants of the United States and Canada: 204 pp. Comstock Publishing Associates.
- Ipser, R.M., Brinkman, M.A., Gardner, W.A., Peeler, H.B. 2004. A survey of ground-dwelling ants (Hymenoptera: Formicidae) in Georgia. Florida Entomologist 87: 253-260.
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87 (doi:10.3897@jhr.70.35207).
- MacGown, J.A., Booher, D., Richter, H., Wetterer, J.K., Hill, J.G. 2021. An updated list of ants of Alabama (Hymenoptera: Formicidae) with new state records. Transactions of the American Entomological Society 147: 961-981 (doi:10.3157/061.147.0409).
- Mayr, G. 1862. Myrmecologische Studien. Verh. K-K. Zool.-Bot. Ges. Wien 12: 649-776 (page 661, worker, queen described)
- Mayr, G. 1886d. Die Formiciden der Vereinigten Staaten von Nordamerika. Verh. K-K. Zool.-Bot. Ges. Wien 36: 419-464 (page 420, Junior synonym of castaneus)
- Oswalt, D.A. 2007. Nesting and foraging characteristics of the black carpenter ant Camponotus pennsylvanicus DeGeer (Hymenoptera: Formicidae). Ph.D. thesis, Clemson University.
- Rafiqi, A.M., Rajakumar, A., Abouheif, E. 2020. Origin and elaboration of a major evolutionary transition in individuality. Nature 585, 239–244. (doi:10.1038/s41586-020-2653-6).
- Santschi, F. 1936b. Contribution à l'étude des fourmis de l'Amérique du Sud. Rev. Entomol. (Rio J.) 6: 196-218.
- Santschi, F. 1937h. Fourmis du Japon et de Formose. Bull. Ann. Soc. Entomol. Belg. 77: 361-388.
- Shrestha B, Tanaka E, Hyun MW, Han JG, Kim CS, Jo JW, Han SK, Oh J, Sung JM, Sung GH. 2017. Mycosphere Essay 19. Cordyceps species parasitizing hymenopteran and hemipteran insects. Mycosphere 8(9): 1424–1442 (DOI 10.5943/mycosphere/8/9/8).
- Smith, D. R. 1979. Superfamily Formicoidea. Pp. 1323-1467 in: Krombein, K. V., Hurd, P. D., Smith, D. R., Burks, B. D. (eds.) Catalog of Hymenoptera in America north of Mexico. Volume 2. Apocrita (Aculeata). Washington, D.C.: Smithsonian Institution Press, pp. i-xvi, 1199-2209.
- Waters, J.S., Keough, N.W., Burt, J., Eckel, J.D., Hutchinson, T., Ewanchuk, J., Rock, M., Markert, J.A., Axen, H.J., Gregg, D. 2022. Survey of ants (Hymenoptera, Formicidae) in the city of Providence (Rhode Island, United States) and a new northern-most record for Brachyponera chinensis (Emery, 1895). Check List 18(6), 1347–1368 (doi:10.15560/18.6.1347).
- Wheeler, G. C.; Wheeler, J. 1953e. The ant larvae of the subfamily Formicinae. Part II. Ann. Entomol. Soc. Am. 46: 175-217.
- Wheeler, G. C.; Wheeler, J. 1968a. The ant larvae of the subfamily Formicinae (Hymenoptera: Formicidae): supplement. Ann. Entomol. Soc. Am. 61: 205-222 (page 216, larva described)
- Wheeler, W. M. 1910g. The North American ants of the genus Camponotus Mayr. Ann. N. Y. Acad. Sci. 20: 295-354 (page 317, soldier, worker, queen, male described)
- Wheeler, W. M. 1913d. Ants collected in Georgia by Dr. J. C. Bradley and Mr. W. T. Davis. Psyche (Camb.) 20: 112-117 (page 117, Revived from synonymy as subspecies of castaneus)
- Wheeler, W. M. 1917k. A list of Indiana ants. Proc. Indiana Acad. Sci. 26: 460-466 (page 465, Revived from synonymy as subspecies of castaneus)
- Wheeler, W. M. 1932a. A list of the ants of Florida with descriptions of new forms. J. N. Y. Entomol. Soc. 40: 1-17 (page 13, Revived from synonymy as subspecies of castaneus)
- Yanoviak, S.P., Frederick, D.N. 2014. Water surface locomotion in tropical canopy ants. Journal of Experimental Biology 217, 2163–2170 (doi:10.1242/jeb.101600).
References based on Global Ant Biodiversity Informatics
- Beckmann R. L., and J. M. Stucky. 1981. Extrafloral Nectaries and Plant Guarding in Ipomoea pandurata (L.) G. F. W. Mey. (Convolvulaceae). American Journal of Botany 68(1): 72-79.
- Belcher A. K., M. R. Berenbaum, and A. V. Suarez. 2016. Urbana House Ants 2.0.: revisiting M. R. Smith's 1926 survey of house-infesting ants in central Illinois after 87 years. American Entomologist 62(3): 182-193.
- Campbell J. W., S. M. Grodsky, D. A. Halbritter, P. A. Vigueira, C. C. Vigueira, O. Keller, and C. H. Greenberg. 2019. Asian needle ant (Brachyponera chinensis) and woodland ant responses to repeated applications of fuel reduction methods. Ecosphere 10(1): e02547.
- Clark A. T., J. J. Rykken, and B. D. Farrell. 2011. The Effects of Biogeography on Ant Diversity and Activity on the Boston Harbor Islands, Massachusetts, U.S.A. PloS One 6(11): 1-13.
- Cokendolpher J. C., and O. F. Francke. 1990. The ants (Hymenoptera, Formicidae) of western Texas. Part II. Subfamilies Ecitoninae, Ponerinae, Pseudomyrmecinae, Dolichoderinae, and Formicinae. Special Publications, the Museum. Texas Tech University 30:1-76.
- Coovert G. A. 2005. The Ants of Ohio (Hymenoptera: Formicidae). Ohio Biological Survey, Inc. 15(2): 1-207.
- Coovert, G.A. 2005. The Ants of Ohio (Hymenoptera: Formicidae) Ohio Biological Survey Bulletin New Series Volume 15(2):1-196
- Dash S. T. and L. M. Hooper-Bui. 2008. Species diversity of ants (Hymenoptera: Formicidae) in Louisiana. Conservation Biology and Biodiversity. 101: 1056-1066
- Davis W. T., and J. Bequaert. 1922. An annoted list of the ants of Staten Island and Long Island, N. Y. Bulletin of the Brooklyn Entomological Society 17(1): 1-25.
- Del Toro I., K. Towle, D. N. Morrison, and S. L. Pelini. 2013. Community Structure, Ecological and Behavioral Traits of Ants (Hymenoptera: Formicidae) in Massachusetts Open and Forested Habitats. Northeastern Naturalist 20: 1-12.
- Del Toro, I. 2010. PERSONAL COMMUNICATION. MUSEUM RECORDS COLLATED BY ISRAEL DEL TORO
- Deyrup M., C. Johnson, G. C. Wheeler, J. Wheeler. 1989. A preliminary list of the ants of Florida. Florida Entomologist 72: 91-101
- DuBois M. B. 1981. New records of ants in Kansas, III. State Biological Survey of Kansas. Technical Publications 10: 32-44
- Dubois, M.B. and W.E. Laberge. 1988. An Annotated list of the ants of Illionois. pages 133-156 in Advances in Myrmecology, J. Trager
- Ellison A. M., and E. J. Farnsworth. 2014. Targeted sampling increases knowledge and improves estimates of ant species richness in Rhode Island. Northeastern Naturalist 21(1): NENHC-13NENHC-24.
- Emery C. 1893. Beiträge zur Kenntniss der nordamerikanischen Ameisenfauna. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 7: 633-682.
- Emery C. 1895. Beiträge zur Kenntniss der nordamerikanischen Ameisenfauna. (Schluss). Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 8: 257-360.
- Epperson, D.M. and C.R. Allen. 2010. Red Imported Fire Ant Impacts on Upland Arthropods in Southern Mississippi. American Midland Naturalist, 163(1):54-63.
- Forster J.A. 2005. The Ants (hymenoptera: Formicidae) of Alabama. Master of Science, Auburn University. 242 pages.
- General D., and L. Thompson. 2008. Ants of Arkansas Post National Memorial: How and Where Collected. Journal of the Arkansas Academy of Science 62: 52-60.
- General D., and L. Thompson. 2008. New distributional records of ants in Arkansas. Journal of the Arkansas Academy of Science 62: 148-150.
- General D.M. & Thompson L.C. 2007. Ants (Hymenoptera: Formicidae) of Arkansas Post National Memorial. Journal of the Arkansas Acaedemy of Science. 61: 59-64
- Guénard B., K. A. Mccaffrey, A. Lucky, and R. R. Dunn. 2012. Ants of North Carolina: an updated list (Hymenoptera: Formicidae). Zootaxa 3552: 1-36.
- Hayes W. P. 1925. A preliminary list of the ants of Kansas (Hymenoptera, Formicidae). [concl.]. Entomological News 36: 69-73
- Herbers J. N. 1989. Community structure in north temperate ants: temporal and spatial variation. Oecologia 81: 201-211.
- IZIKO South Africa Museum Collection
- Ipser R. M. 2004. Native and exotic ants (Hymenoptera: Formicidae) of Georgia: Ecological Relationships with implications for development of biologically-based management strategies. Doctor of Philosophy thesis, University of Georgia. 165 pages.
- Ipser, R.M., M.A. Brinkman, W.A. Gardner and H.B. Peeler. 2004. A Survey of Ground-Dwelling Ants (Hymenoptera: Formicidae) in Georgia. The Florida Entomologist 87(3) 253-260.
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87.
- Ivanov K., L. Hightower, S. T. Dash, and J. B. Keiper. 2019. 150 years in the making: first comprehensive list of the ants (Hymenoptera: Formicidae) of Virginia, USA. Zootaxa 4554 (2): 532–560.
- Jeanne R. J. 1979. A latitudinal gradient in rates of ant predation. Ecology 60(6): 1211-1224.
- Lubertazi, D. Personal Communication. Specimen Data from Museum of Comparative Zoology at Harvard
- Lynch J. F. 1988. An annotated checklist and key to the species of ants (Hymenoptera: Formicidae) of the Chesapeake Bay region. The Maryland Naturalist 31: 61-106
- MacGown J. A., J. G. Hill, R. L. Brown, T. L. Schiefer, J. G. Lewis. 2012. Ant diversity at Noxubee National Wildlife Refuge in Oktibbeha, Noxubee, and Winston Counties, Mississippi. Mississippi Agricultural and Forestry Experiment Station Bulletin 1197: 1-30
- MacGown J. A., J. G. Hill, and R. L. Brown. 2010. Native and exotic ant in Mississippi state parks. Proceedings: Imported Fire Ant Conference, Charleston, South Carolina, March 24-26, 2008: 74-80.
- MacGown J. A., R. L. Brown, J. G. Hill, and B. Layton. 2007. Carpenter ants of Mississippi. Mississippi Agricultural and Forestry Experiment Station Bulletin 1158: 1-35.
- MacGown J. A., and R. L. Brown. 2006. Survey of the ants (Hymenoptera: Formicidae) of the Tombigbee National Forest in Mississippi. Journal of the Kansas Entomological Society 79(4):325-340.
- MacGown, J.A and J.A. Forster. 2005. A preliminary list of the ants (Hymenoptera: Formicidae) of Alabama, U.S.A. Entomological News 116(2):61-74
- MacGown, J.A. and JV.G. Hill. Ants of the Great Smoky Mountains National Park (Tennessee and North Carolina).
- MacGown, J.A. and R.L. Brown. 2006. Survey of the ants (Hymenoptera: Formicidae) of the Tombigbee National Forest in Mississippi. Journal of the Kansas Entomological Society 79(4):325-340.
- MacGown, J.A., J.G. Hill, R.L. Brown and T.L. 2009. Ant Diversity at Noxubee National Wildlife Refuge in Oktibbeha, Noxubee, and Winston Counties, Mississippi Report #2009-01. Schiefer. 2009.
- MacGown. J. 2011. Ants collected during the 25th Annual Cross Expedition at Tims Ford State Park, Franklin County, Tennessee
- Macgown J. A., S. Y. Wang, J. G. Hill, and R. J. Whitehouse. 2017. A List of Ants (Hymenoptera: Formicidae) Collected During the 2017 William H. Cross Expedition to the Ouachita Mountains of Arkansas with New State Records. Transactions of the American Entomological Society, 143(4): 735-740.
- Martelli, M.G., M.M. Ward and Ann M. Fraser. 2004. Ant Diversity Sampling on the Southern Cumberland Plateau: A Comparison of Litter Sifting and Pitfall Trapping. Southeastern Naturalist 3(1): 113-126
- McClelland L. A. 1978. The Nebraska distribution of the ant genus Camponotus Mayr (Hymenoptera: Formicidae). Master's Thesis, Department of Biology and the faculty of the Graduate of Nebraska at Omaha, 72 pages.
- Menke S. B., E. Gaulke, A. Hamel, and N. Vachter. 2015. The effects of restoration age and prescribed burns on grassland ant community structure. Environmental Entomology http://dx.doi.org/10.1093/ee/nvv110
- Menzel T. O., and T. E. Nebeker. 2008. Distribution of Hybrid Imported Fire Ants (Hymenoptera: Formicidae) and Some Native Ant Species in Relation to Local Environmental Conditions and Interspecific Competition in Mississippi Forests. Ann. Entomol. Soc. Am. 101(1): 119-127.
- Morrison, L.W. 2002. Long-Term Impacts of an Arthropod-Community Invasion by the Imported Fire Ant, Solenopsis invicta. Ecology 83(8):2337-2345
- Nuhn, T.P. and C.G. Wright. 1979. An Ecological Survey of Ants (Hymenoptera: Formicidae) in a Landscaped Suburban Habitat. American Midland Naturalist 102(2):353-362
- O'Keefe S. T., J. L. Cook, T. Dudek, D. F. Wunneburger, M. D. Guzman, R. N. Coulson, and S. B. Vinson. 2000. The Distribution of Texas Ants. The Southwestern Entomologist 22: 1-92.
- O'Neill J.C. and Dowling A.P.G. 2011. A Survey of the Ants (hymenoptera: Formicidae) of Arkansas and the Ozark Mountains. An Undergraduate Honors, University of Arkansas. 18pages.
- Sargent J. M., Benson. E.P., Zungoli. P. A. and Bridges. W. C. 2002. Carpenter Ant (Hymenoptera: Formicidae) Fauna of South Carolina. J. Agric. Urban Entomol. 18: 227-236
- Smith M. R. 1924. An annotated list of the ants of Mississippi (Hym.) (continued from page 85). Entomological News 35: 121-127.
- Smith M. R. 1935. A list of the ants of Oklahoma (Hymen.: Formicidae) (continued from page 241). Entomological News 46: 261-264.
- Sturtevant A. H. 1931. Ants collected on Cape Cod, Massachusetts. Psyche (Cambridge) 38: 73-79
- Talbot M. 1976. A list of the ants (Hymenoptera: Formicidae) of the Edwin S. George Reserve, Livingston County, Michigan. Great Lakes Entomologist 8: 245-246.
- Toennisson T. A., N. J. Sanders, W. E. Klingeman, and K. M. Vail. 2011. Influences on the Structure of Suburban Ant (Hymenoptera: Formicidae) Communities and the Abundance of Tapinoma sessile. Environ. Entomol. 40(6): 1397-1404.
- Turner C. R., and J. L. Cook. 1998. The ants (Hymenoptera: Formicidae) of the Caddo Lake region of northeast Texas. Texas Journal of Science 50: 171-173.
- Wang C., J. Strazanac and L. Butler. 2000. Abundance, diversity and activity of ants (Hymenoptera: Formicidae) in oak-dominated mixed Appalachian forests treated with microbial pesticides. Environmental Entomology. 29: 579-586
- Warren, L.O. and E.P. Rouse. 1969. The Ants of Arkansas. Bulletin of the Agricultural Experiment Station 742:1-67
- Wheeler G. C., J. N. Wheeler, and P. B. Kannowski. 1994. Checklist of the ants of Michigan (Hymenoptera: Formicidae). The Great Lakes Entomologist 26(4): 297-310
- Wheeler G. C., and J. Wheeler J. 1989. A checklist of the ants of Oklahoma. Prairie Naturalist 21: 203-210.
- Wheeler W. M. 1905. An annotated list of the ants of New Jersey. Bulletin of the American Museum of Natural History. 21: 371-403.
- Wheeler W. M. 1910. The North American ants of the genus Camponotus Mayr. Annals of the New York Academy of Sciences 20: 295-354.
- Wheeler W. M. 1913. Ants collected in Georgia by Dr. J. C. Bradley and Mr. W. T. Davis. Psyche (Cambridge) 20: 112-117.
- Wheeler W. M. 1932. A list of the ants of Florida with descriptions of new forms. J. N. Y. Entomol. Soc. 40: 1-17.
- Wheeler, G.C. and J. Wheeler. 1985. A checklist of Texas ants. Prairie Naturalist 17:49-64.
- Wheeler, G.C., J. Wheeler and P.B. Kannowski. 1994. CHECKLIST OF THE ANTS OF MICHIGAN (HYMENOPTERA: FORMICIDAE). Great Lakes Entomologist 26:1:297-310
- Young J., and D. E. Howell. 1964. Ants of Oklahoma. Miscellaneous Publication. Oklahoma Agricultural Experimental Station 71: 1-42.
- Young, J. and D.E. Howell. 1964. Ants of Oklahoma. Miscellaneous Publications of Oklahoma State University MP-71
- Zettler J. A., M. D. Taylor, C. R. Allen, and T. P. Spira. 2004. Consequences of Forest Clear-Cuts for Native and Nonindigenous Ants (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 97(3): 513-518.
- Pages using DynamicPageList3 parser function
- Photo Gallery
- Need species key
- North temperate
- North subtropical
- Fungus Associate
- Host of Ophiocordyceps unilateralis
- FlightMonth
- Species
- Extant species
- Formicidae
- Formicinae
- Camponotini
- Camponotus
- Camponotus americanus
- Formicinae species
- Camponotini species
- Camponotus species
- Need Overview
- Need Body Text







