Camponotus castaneus
| Camponotus castaneus | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Formicinae |
| Tribe: | Camponotini |
| Genus: | Camponotus |
| Subgenus: | Camponotus |
| Species complex: | herculeanus |
| Species: | C. castaneus |
| Binomial name | |
| Camponotus castaneus (Latreille, 1802) | |
| Synonyms | |
| |
Camponotus castaneus commonly nests in the soil, or under stones (Buren, 1944) or in rotten stumps or logs or fallen branches exposed to the soil. It forms moderately populous colonies but is very timid and apparently primarily nocturnal (Wheeler, 1910a, 1917a). MacGown and Brown (2006) found nests in the soil at the base of Fagus grandifolia in a seepage area. In warm sites, C. castaneus tends to occupy relatively heated chambers (Diamond et al., 2012). (Kackay, 2019)
Photo Gallery
 Camponotus castaneus with the fungus Ophiocordyceps kimflemingiae, in Old Garners Ferry Rd, Rembert, South Carolina, USA.
Camponotus castaneus with the fungus Ophiocordyceps kimflemingiae, in Old Garners Ferry Rd, Rembert, South Carolina, USA.
Identification
The following information is derived from Mackay, New World Carpenter Ants (2019)
Compare with Camponotus americanus, Camponotus semitestaceus
All castes of C. castaneus are usually easily recognized by their deep, golden honey color and that most surfaces are at least partially glossy and shining. The cheeks are often without setae, although two setae may be present on workers and females (in rare instances up to 6 setae may be present on the cheeks, especially in females and minors, but no erect and suberect setae are near the eyes), the sides of the head are nearly always without erect and suberect setae and the scapes are without erect and suberect setae (except at the apex). The base of the scape is slightly flattened. The anterior border of the clypeus of the major and female (and to a lesser extent the minor) is crenulated. The dorsum of the gaster is glossy and shining. The mid and posterior tibiae have a row of erect or suberect setae which extend at least half the length of the flexor surface. The clypeal carina is present, but poorly developed.
The male is similar in color and is relatively large (total length 11 mm).
Comparisons
Camponotus castaneus is difficult to separate from Camponotus americanus (eastern US), which was previously considered to be a subspecies (Creighton, 1950). The most obvious character to separate the two species is the lack of erect or suberect setae on the cheeks (rarely up to six are present) of C. castaneus, whereas the cheeks of majors, minors and females of C. americanus usually have more than eight suberect setae. The males of C. castaneus have no setae on the cheeks, the males of C. americanus usually have two suberect setae.
The Camponotus castaneus major worker superficially resembles that of Camponotus semitestaceus (subgenus Tanaemyrmex, western and south-central US) in color and size but can be easily distinguished as it lacks the lobe or widened area near the base of the scape, which is present in C. semitestaceus, as well as a clypeal carina which is lacking in C. castaneus.
Distribution
The following information is derived from Mackay, New World Carpenter Ants (2019)
Camponotus castaneus was collected from cleared upland deciduous forest along a power line, in oak and long-leaf pine sand barrens, at the edge of an old field, in saw palmetto, in dry oak forests, long leaf pine forests, mixed hardwood forests, mixed pine hardwoods, on a flood plain, in sand pine/rosemary scrub, sand pine/turkey oak, in a recently burned hardwood forest, in burned loblolly pine forest, second growth pines, and even desert thorn scrub. It was reported from deciduous and mixed forest and are typical residents of savannas (Colby and Prowell, 2006), from hardwood hammock and pine flatwoods (King, 2007), and from urban habitats (Guénard et al., 2015), with increased abundance in disturbed areas (Walker, 2013). It is a warm climate forest dweller (Ellison et al., 2012).
Latitudinal Distribution Pattern
Latitudinal Range: 43.655° to 24.48472222°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Nearctic Region: United States (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
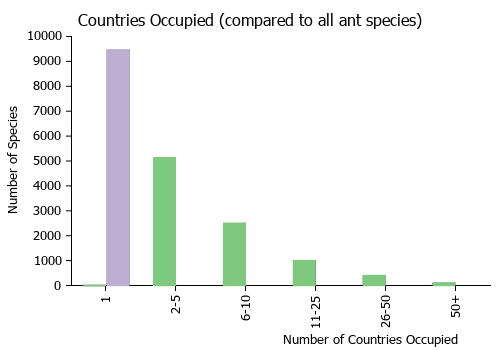
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
|
Biology
The following information is derived from Mackay, New World Carpenter Ants (2019)
Workers forage between 9:00 - 16:00; Ellison et al. (2012) reported they were mainly nocturnal in New England. They are predaceous (collected in live mealworm baits on the soil surface); Ellison et al. (2012) concludes they are omnivorous. They also collect dead insects and tend homopterans. They are important in seed removal, which is affected by temperature (Stuble et al., 2014).
Sexuals overwinter in the nest and fly the following year. Sexuals were collected in nests in February, March, April, June and August. Nuptial flights occur at night when sexuals can be collected at lights from April - September. Loose sexuals were collected loose on ground from April - September and November.
Camponotus castaneus is a host of the endosymbiotic proteobacteria Candidatus Blochmannia (Degnan et al., 2004), which has close relatives that occur in aphids and the tsetse fly (Sauer et al., 2000). It is also the host of Wolbachia bacteria (Wernegreen et al., 2009). They have gram-negative prokaryotic endosymbionts in the follicle cells (Peloquin et al., 2001).
It is infected by the fungus Ophiocordyceps unilateralis sensu lato (de Bakker et al., 2014) and parasitized by the nematode Rabbium paradoxus (Poinar, 2012).
Camponotus castaneus is a host of the ant cricket Myrmecophilus pergandei (Hebard, 1920).
Camponotus castaneus occasionally forages nocturnally into structures (Hansen and Klotz, 2005; Ellison et al., 2012), but is generally not a major economic pest. It is one of the four most common house infesting ants in Illinois (Walker, 2013).
Hölldobler and Engel-Siegel (1984) discussed the lack of the metapleural gland in this species. Hohl et al. (2003) characterized the trail pheromones and Dufour gland contents. Kohl et al. (2003) identified the trail pheromone of Camponotus castaneus as 6-sec-butyl-2,5-dimethyltetrahydro-2H-pyran-2-one 124 (Morgan, 2008).
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a host for the cricket Myrmecophilus pergandei (a myrmecophile) in United States.
- This species is a host for the braconid wasp Elasmosoma pergandei (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
Fungi
- This species is a host for the fungus Ophiocordyceps unilateralis (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
- This species is a host for the fungus Ophiocordyceps kimflemingiae (a pathogen) (Araujo et al., 2018).
- This species is a host for the fungus Ophiocordyceps unilateralis sensu lato (a pathogen) (Mangold et al., 2019).
Mangold et al. (2019) studied the mechanistic details of how the fungus infects its worker hosts and causes the ants to clamp their mandibles onto a leaf with a so called death grip. This anchoring attaches the dying worker to the vegetation, which in turn allows the fungus to develop its spores in a location where their release will occur over and thus onto the forest floor below. This study found the fungus builds and penetrates around cells and tissues of the ant's mandibular muscles to produce its death grip. Motor neurons and neuromuscular junctions did not show any evidence of being effected or attacked by the fungus.
Nematodes
- This species is a host for the nematode Rabbium paradoxus (a parasite) in Florida, United States (Poinar et al., 1989).
Flight Period
| X | X | X | X | X | |||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
Source: antkeeping.info.
- Check details at Worldwide Ant Nuptial Flights Data, AntNupTracker and AntKeeping.
- Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Castes
Worker
Images from AntWeb
   
| |
| Worker. Specimen code casent0103654. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
   
| |
| Worker. Specimen code casent0104761. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by UCDC, Davis, CA, USA. |
   
| |
| Worker. Specimen code casent0104977. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by UCDC, Davis, CA, USA. |
   
| |
| Worker (major/soldier). Specimen code casent0172603. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by LACM, Los Angeles, CA, USA. |
   
| |
| Worker (major/soldier). Specimen code casent0172604. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by LACM, Los Angeles, CA, USA. |
   
| |
| Worker. Specimen code casent0103653. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
Queen
Images from AntWeb
    
| |
| Queen (alate). Specimen code CASENT0103656. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
Male
Images from AntWeb
    
| |
| Male. Specimen code CASENT0103657. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- castaneus. Formica castanea Latreille, 1802c: 118, pl. 3, figs. 11, 12 (w.q.m.) U.S.A. (Pennsylvania, Carolina).
- Wheeler, W.M. 1910d: 321 (s.).
- Combination in Camponotus: Roger, 1863b: 6;
- combination in C. (Tanaemyrmex): Creighton, 1950a: 375;
- combination in C. (Camponotus): Forel, 1914a: 266; Mackay, 2019: 182.
- Junior synonym of herculeanus: Smith, F. 1858b: 53; Mayr, 1863: 399; Forel, 1874: 96 (in list); Emery & Forel, 1879: 447.
- Status as species: Lepeletier de Saint-Fargeau, 1835: 215; Haliday, 1836: 329; Mayr, 1886d: 420; Forel, 1886f: 141; Cresson, 1887: 255; Dalla Torre, 1893: 223; Emery, 1893i: 673; Emery, 1896d: 372 (in list); Wheeler, W.M. 1905f: 402; Wheeler, W.M. 1906b: 22; Wheeler, W.M. 1910d: 321 (redescription); Wheeler, W.M. 1910g: 571; Wheeler, W.M. 1913c: 117; Forel, 1914a: 266; Forel, 1914d: 288; Wheeler, W.M. 1916m: 600; Wheeler, W.M. 1917i: 465; Emery, 1925b: 74; Smith, M.R. 1930a: 6; Wheeler, W.M. 1932a: 13; Dennis, 1938: 300; Wesson, L.G. & Wesson, R.G. 1940: 103; Buren, 1944a: 294; Creighton, 1950a: 375; Smith, M.R. 1951a: 839; Smith, M.R. 1958c: 143; Carter, 1962a: 7 (in list); Smith, D.R. 1979: 1428; DuBois & LaBerge, 1988: 147; Deyrup, et al. 1989: 100; Bolton, 1995b: 91; Deyrup, 2003: 44; Coovert, 2005: 164; Hansen & Klotz, 2005: 95; MacGown & Forster, 2005: 65; MacGown, et al. 2007: 19; Ellison, et al. 2012: 119; Deyrup, 2017: 187; Mackay, 2019: 182 (redescription).
- Senior synonym of clarus: Mayr, 1886d: 420; Forel, 1886f: 141; Cresson, 1887: 255; Dalla Torre, 1893: 223; Emery, 1896d: 372; Smith, M.R. 1951a: 839; Smith, D.R. 1979: 1428; Bolton, 1995b: 91; Mackay, 2019: 182.
- Senior synonym of mellea: Mayr, 1886d: 420; Forel, 1886f: 141; Cresson, 1887: 255; Dalla Torre, 1893: 223; Emery, 1896d: 372; Wheeler, W.M. 1906b: 22; Wheeler, W.M. 1910d: 321; Emery, 1925b: 74; Creighton, 1950a: 375; Smith, M.R. 1951a: 839; Smith, D.R. 1979: 1428; Bolton, 1995b: 91; Mackay, 2019: 182.
- clarus. Camponotus clarus Mayr, 1862: 660 (w.) U.S.A. (Pennsylvania).
- Status as species: Roger, 1863b: 5; Mayr, 1863: 398.
- Junior synonym of mellea: Mayr, 1866a: 485 (in text).
- Junior synonym of castaneus: Mayr, 1886d: 420; Forel, 1886f: 141; Cresson, 1887: 255; Dalla Torre, 1893: 223; Emery, 1896d: 372; Smith, M.R. 1951a: 839; Smith, D.R. 1979: 1428; Bolton, 1995b: 91; Mackay, 2019: 182.
- mellea. Formica mellea Say, 1836: 286 (m.) U.S.A. (Louisiana).
- Mayr, 1866a: 485 (w.q.).
- Combination in Camponotus: Roger, 1863b: 5.
- Status as species: Smith, F. 1858b: 54; Roger, 1863b: 5; Mayr, 1866a: 485; Forel, 1879a: 60.
- Junior synonym of castaneus: Mayr, 1886d: 420; Forel, 1886f: 141; Cresson, 1887: 255; Dalla Torre, 1893: 223; Emery, 1896d: 372; Wheeler, W.M. 1906b: 22; Wheeler, W.M. 1910d: 321; Emery, 1925b: 74; Creighton, 1950a: 375; Smith, M.R. 1951a: 839; Smith, D.R. 1979: 1428; Bolton, 1995b: 111; Mackay, 2019: 182.
Type Material
Wheeler (1910) - The types of this species, which is easily recognized by the red color of all the phases, came from the Carolinas and Pennsylvania. Types were not examined by Mackay (2019).
Description
The following information is derived from Mackay, New World Carpenter Ants (2019)
Major worker measurements (mm): HL 2.88 - 3.38, HW 2.62 - 3.40, SL 3.10 - 3.25, EL 0.61 - 0.71, CL 0.86 - 1.09, CW 1.10 - 1.20, WL 4.20 - 4.66, FFL 2.62 - 2.86, FFW 0.68 - 0.79. Indices: CI 91 - 101, SI 96 - 108, CLI 110 - 128, FFI 26 - 28.
Mandibles with 5 teeth; anterior border of clypeus convex, crenulate, clypeus convex, but with carina poorly marked; head heart-shaped, with anterior half strongly narrowed; posterior margin concave; eyes failing to reach sides of head by nearly 1 minimum diameter; scape extending past posterior lateral corner of head by 2 funicular segments; dorsopropodeum about 1½ times length of posteropropodeum; petiole with node rounded as seen from front.
Erect and suberect setae scattered on dorsal and ventral surfaces of head, clypeus, nearly absent on cheeks and sides of head, present on mesosoma, petiole and gaster; appressed pubescence very tiny and sparse.
Sculpture coriaceous, mostly shining, especially gaster.
Color light brown, ranging to dark brown in a few specimens.
Minor worker measurements (mm): HL 1.94 - 2.58, HW 1.34 - 2.10, SL 2.56 - 2.88, EL 0.49 - 0.59, CL 0.54 - 0.74, CW 0.80 - 1.00, WL 3.08 - 3.82, FFL 2.06 - 2.44, FFW 0.48 - 0.61. Indices: CI 69 - 81, SI 112 - 132, CLI 136 - 149, FFI 23 - 25.
Mandibles with 5 teeth; anterior border of clypeus similar to that of major, except with poorly developed crenulations; posterior margin convex; eyes not reaching (larger minors) or barely surpassing (smaller minors) sides of head; scape extending 3 funicular segments (larger minor workers) to ½ length (smallest minor workers) past posterior lateral corner of head; dorsopropodeum twice length of posteropropodeum; petiole thick in profile, petiolar apex round as seen from front.
Erect and suberect setae, appressed pubescence, sculpture and color as in major.
Female measurements (mm): HL 3.26 - 3.38, HW 3.06 - 3.36, SL 2.92 - 3.02, EL 0.79 - 0.86, CL 0.96 - 1.01, CW 1.19 - 1.26, WL 5.48 - 6.00, FFL 2.88 - 3.00, FFW 0.73 - 0.74. Indices: CI 94 - 99, SI 89 - 90, CLI 123 - 125, FFI 24 - 25.
Mandible with 5 teeth, similar to major in most aspects, except head not as strongly narrowed anteriorly; clypeus with crenulations along anterior border; eyes nearly reaching sides of head.
Erect and suberect setae as in major, but more commonly found on cheeks and even sides of head; appressed pubescence, sculpture and color as in major.
Male measurements (mm): HL 1.58 - 1.74, HW 1.34 - 1.54, SL 2.14 - 2.28, EL 0.58 - 0.61, CL 0.48 - 0.51, CW 0.66 - 0.93, WL 3.92 - 4.14, FFL 2.52 - 2.68, FFW 0.43 - 0.51. Indices: CI 85 - 89, SI 131 - 136, CLI 139 - 180, FFI 17 - 19.
Mandible without teeth (other than apical angle); medial anterior border of clypeus nearly straight, without crenulations, clypeus with convex surface, nearly without evidence of carina; scape extending more than ½ length past posterior lateral corner of head; propodeum rounded; petiole thick, petiolar apex weakly convex as seen from behind (rarely concave).
Erect and suberect setae, appressed pubescence, sculpture and color as in major.
References
- Mayr, G. 1866a. Myrmecologische Beiträge. Sitzungsberichte der k. Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Classe 53: 484-517.
- Araújo, J.P.M., Evans, H.C., Kepler, R., Hughes, D.P. 2018. Zombie-ant fungi across continents: 15 new species and new combinations within Ophiocordyceps. I. Myrmecophilous hirsutelloid species. Studies in Mycology 90: 119–160 (DOI 10.1016/j.simyco.2017.12.002).
- Carroll, T.M. 2011. The ants of Indiana (Hymenoptera: Formicidae). M.S. thesis, Purdue University.
- Creighton, W. S. 1950a. The ants of North America. Bulletin of the Museum of Comparative Zoology 104: 1-585 (page 375, Combination in C. (Tanaemyrmex))
- Cushing, P.E. 2012. Spider-ant associations: An updated review of myrmecomorphy, myrmecophily, and myrmecophagy in spiders. Psyche: A Journal of Entomology 2012, 1–23 (doi:10.1155/2012/151989).
- Davis, T. 2009. The ants of South Carolina (thesis, Clemson University).
- de Bekker, C., Beckerson, W.C., Elya, C. 2021. Mechanisms behind the madness: How do zombie-making fungal entomopathogens affect host behavior to increase transmission? mBio, e01872–21 (doi:10.1128/mbio.01872-21).
- de Bekker, C., Will, I., Das, B., Adams, R.M.M. 2018. The ants (Hymenoptera: Formicidae) and their parasites: effects of parasitic manipulations and host responses on ant behavioral ecology. Myrmecological News 28: 1-24 (doi:10.25849/myrmecol.news_028:001).
- Ellison, A. M., N. J. Gotelli, E. J. Farnsworth, and G. D. Alpert. 2012. A Field Guide to the Ants of New England. Yale University Press, New Haven.
- Emery, C. 1920b. Le genre Camponotus Mayr. Nouvel essai de la subdivision en sous-genres. Rev. Zool. Afr. (Bruss.) 8: 229-260.
- Emery, C. 1925d. Hymenoptera. Fam. Formicidae. Subfam. Formicinae. Genera Insectorum 183: 1-302.
- Forel, A. 1886h. Études myrmécologiques en 1886. Ann. Soc. Entomol. Belg. 30: 131-215 (page 141, Senior synonym of mella (and its junior synonym clarus))
- Forel, A. 1914a. Le genre Camponotus Mayr et les genres voisins. Rev. Suisse Zool. 22: 257-276 (page 266, Combination in C. (Camponotus))
- Gochnour, B.M., Suiter, D.R., Booher, D. 2019. Ant (Hymenoptera: Formicidae) fauna of the Marine Port of Savannah, Garden City, Georgia (USA). Journal of Entomological Science 54, 417-429 (doi:10.18474/jes18-132).
- Guénard, B., Silverman, J. 2011. Tandem carrying, a new foraging strategy in ants: description, function, and adaptive significance relative to other described foraging strategies. Naturwissenschaften 98(8), 651–659 (doi:10.1007/s00114-011-0814-z).
- Hansen, L.D. & Klotz, J.H. 2005. Carpenter ants of the United States and Canada: 204 pp. Comstock Publishing Associates.
- Hill, J.G. 2015. Ants (Hymenoptera: Formicidae) of the Big Thicket Region of Texas. Midsouth Entomologist 8: 24-34.
- Ipser, R.M., Brinkman, M.A., Gardner, W.A., Peeler, H.B. 2004. A survey of ground-dwelling ants (Hymenoptera: Formicidae) in Georgia. Florida Entomologist 87: 253-260.
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87 (doi:10.3897@jhr.70.35207).
- Laciny, A. 2021. Among the shapeshifters: parasite-induced morphologies in ants (Hymenoptera, Formicidae) and their relevance within the EcoEvoDevo framework. EvoDevo 12, 2 (doi:10.1186/s13227-021-00173-2).
- Latreille, P.A. 1802. Histoire naturelle des fourmis, et recueil de mémoires et d'observations sur les abeilles, les araignées, les faucheurs, et autres insectes. Paris: Impr. Crapelet (chez T. Barrois), xvi + 445 pp.
- MacGown, J.A., Booher, D., Richter, H., Wetterer, J.K., Hill, J.G. 2021. An updated list of ants of Alabama (Hymenoptera: Formicidae) with new state records. Transactions of the American Entomological Society 147: 961-981 (doi:10.3157/061.147.0409).
- Malagocka, J., Eilenberg, J., Jensen, A.B. 2019. Social immunity behaviour among ants infected by specialist and generalist fungi. Current Opinion in Insect Science 33, 99–104 (doi:10.1016/j.cois.2019.05.001).
- Mangold, C. A., M. J. Ishler, R. G. Loreto, M. L. Hazen, and D. P. Hughes. 2019. Zombie ant death grip due to hypercontracted mandibular muscles. The Journal of Experimental Biology. 222:jeb200683. doi:10.1242/jeb.200683
- Mayr, G. 1886d. Die Formiciden der Vereinigten Staaten von Nordamerika. Verh. K-K. Zool.-Bot. Ges. Wien 36: 419-464 (page 420, Senior synonym of mella (and its junior synonym clarus))
- Moura, M.N., Cardoso, D.C., Cristiano, M.P. 2020. The tight genome size of ants: diversity and evolution under ancestral state reconstruction and base composition. Zoological Journal of the Linnean Society, zlaa135 (doi:10.1093/zoolinnean/zlaa135).
- Oswalt, D.A. 2007. Nesting and foraging characteristics of the black carpenter ant Camponotus pennsylvanicus DeGeer (Hymenoptera: Formicidae). Ph.D. thesis, Clemson University.
- Poinar, G., Chabaud, A.G., Bain, O. 1989. Rabbium paradoxus sp. n. (Seuratidae: Skrjabinelazienae) maturing in Camponotus castaneus (Hymenoptera: Forimicidae). Proceedings of the Helminthological Society of Washington 56: 120–124.
- Poinar, G., Jr. 2012. Nematode parasites and associates of ants: Past and present. Psyche, 2012, Article ID 192017, 13 pages (DOI 10.1155/2012/192017).
- Rafiqi, A.M., Rajakumar, A., Abouheif, E. 2020. Origin and elaboration of a major evolutionary transition in individuality. Nature 585, 239–244. (doi:10.1038/s41586-020-2653-6).
- Roger, J. 1863b. Verzeichniss der Formiciden-Gattungen und Arten. Berl. Entomol. Z. 7(B Beilage: 1-65 (page 6, Combination in Camponotus)
- Smith, D. R. 1979. Superfamily Formicoidea. Pp. 1323-1467 in: Krombein, K. V., Hurd, P. D., Smith, D. R., Burks, B. D. (eds.) Catalog of Hymenoptera in America north of Mexico. Volume 2. Apocrita (Aculeata). Washington, D.C.: Smithsonian Institution Press, pp. i-xvi, 1199-2209.
- Smith, M. R. 1947f. A generic and subgeneric synopsis of the United States ants, based on the workers. Am. Midl. Nat. 37: 521-647.
- Smith, M. R. 1965. House-infesting ants of the eastern United States. Their recognition, biology, and economic importance. U. S. Dep. Agric. Tech. Bull. 1326: 1-105.
- Trinh, T., Ouellette, R., de Bekker, C. 2021. Getting lost: the fungal hijacking of ant foraging behaviour in space and time. Animal Behaviour (doi:10.1016/j.anbehav.2021.09.003).
- Waters, J.S., Keough, N.W., Burt, J., Eckel, J.D., Hutchinson, T., Ewanchuk, J., Rock, M., Markert, J.A., Axen, H.J., Gregg, D. 2022. Survey of ants (Hymenoptera, Formicidae) in the city of Providence (Rhode Island, United States) and a new northern-most record for Brachyponera chinensis (Emery, 1895). Check List 18(6), 1347–1368 (doi:10.15560/18.6.1347).
- Wetterer, J.K., Espadaler, X., Ashmole, N.P., Mendel, H., Cutler, C., Endeman, J. 2007. Ants (Hymenoptera: Formicidae) of the South Atlantic islands of Ascension Island, St Helena, and Tristan da Cunha. Myrmecological News 10: 29-37.
- Wheeler, W. M. 1905j. An annotated list of the ants of New Jersey. Bulletin of the American Museum of Natural History. 21: 371-403.
- Wheeler, W. M. 1910g. The North American ants of the genus Camponotus Mayr. Ann. N. Y. Acad. Sci. 20: 295-354 (page 321, soldier described)
- Wheeler, W. M. 1917a. The mountain ants of western North America. Proc. Am. Acad. Arts Sci. 52: 457-569.
- Will, I., Das, B., Trinh, T., Brachmann, A., Ohm, R.A., de Bekker, C. 2020. Genetic underpinnings of host manipulation by Ophiocordyceps as revealed by comparative transcriptomics. G3 Genes|Genomes|Genetics 10, 2275–2296 (doi:10.1534/g3.120.401290).
References based on Global Ant Biodiversity Informatics
- Annotated Ant Species List Ordway-Swisher Biological Station. Downloaded at http://ordway-swisher.ufl.edu/species/os-hymenoptera.htm on 5th Oct 2010.
- Atchison R. A., J. Hulcr, and A. Lucky. 2018. Managed fire frequency significantly influences the litter arthropod community in longleaf pine flatwoods. Environmental Entomology 20(10): 1-11.
- Belcher A. K., M. R. Berenbaum, and A. V. Suarez. 2016. Urbana House Ants 2.0.: revisiting M. R. Smith's 1926 survey of house-infesting ants in central Illinois after 87 years. American Entomologist 62(3): 182-193.
- Colby, D. and D. Prowell. 2006. Ants (Hymenoptera: Formicidae) in Wet Longleaf Pine Savannas in Louisiana. Florida Entomologist 89(2):266-269
- Coovert G. A. 2005. The Ants of Ohio (Hymenoptera: Formicidae). Ohio Biological Survey, Inc. 15(2): 1-207.
- Coovert, G.A. 2005. The Ants of Ohio (Hymenoptera: Formicidae) Ohio Biological Survey Bulletin New Series Volume 15(2):1-196
- Dash S. T. and L. M. Hooper-Bui. 2008. Species diversity of ants (Hymenoptera: Formicidae) in Louisiana. Conservation Biology and Biodiversity. 101: 1056-1066
- Davis W. T., and J. Bequaert. 1922. An annoted list of the ants of Staten Island and Long Island, N. Y. Bulletin of the Brooklyn Entomological Society 17(1): 1-25.
- Del Toro, I. 2010. PERSONAL COMMUNICATION. MUSEUM RECORDS COLLATED BY ISRAEL DEL TORO
- Deyrup M. 2016. Ants of Florida: identification and natural history. CRC Press, 423 pages.
- Deyrup M., C. Johnson, G. C. Wheeler, J. Wheeler. 1989. A preliminary list of the ants of Florida. Florida Entomologist 72: 91-101
- Deyrup, M. and J. Trager. 1986. Ants of the Archbold Biological Station, Highlands County, Florida (Hymenoptera: Formicidae). Florida Entomologist 69(1):206-228
- Dubois, M.B. and W.E. Laberge. 1988. An Annotated list of the ants of Illionois. pages 133-156 in Advances in Myrmecology, J. Trager
- Ellison A. M., and E. J. Farnsworth. 2014. Targeted sampling increases knowledge and improves estimates of ant species richness in Rhode Island. Northeastern Naturalist 21(1): NENHC-13NENHC-24.
- Forster J.A. 2005. The Ants (hymenoptera: Formicidae) of Alabama. Master of Science, Auburn University. 242 pages.
- Frye J. A., T. Frye, and T. W. Suman. 2014. The ant fauna of inland sand dune communities in Worcester County, Maryland. Northeastern Naturalist, 21(3): 446-471.
- General D. M., and L. C. Thompson. 2011. New Distributional Records of Ants in Arkansas for 2009 and 2010 with Comments on Previous Records. Journal of the Arkansas Academy of Science 65: 166-168.
- General D., and L. Thompson. 2008. Ants of Arkansas Post National Memorial: How and Where Collected. Journal of the Arkansas Academy of Science 62: 52-60.
- General D., and L. Thompson. 2008. New distributional records of ants in Arkansas. Journal of the Arkansas Academy of Science 62: 148-150.
- General D.M. & Thompson L.C. 2008. New Distributional Records of Ants in Arkansas for 2008. Journal of the Arkansas Academy of Science. 63: 182-184
- Graham, J.H., A.J. Krzysik, D.A. Kovacic, J.J. Duda, D.C. Freeman, J.M. Emlen, J.C. Zak, W.R. Long, M.P. Wallace, C. Chamberlin-Graham, J.P. Nutter and H.E. Balbach. 2008. Ant Community Composition across a Gradient of Disturbed Military Landscapes at Fort Benning, Georgia. Southeastern Naturalist 7(3):429-448
- Guénard B., K. A. Mccaffrey, A. Lucky, and R. R. Dunn. 2012. Ants of North Carolina: an updated list (Hymenoptera: Formicidae). Zootaxa 3552: 1-36.
- Hill J.G. & Brown R. L. 2010. The Ant (Hymenoptera: Formicidae) Fauna of Black Belt Prairie Remnants in Alabama and Mississippi. Southeastern Naturalist. 9: 73-84
- Hill, J.G. 2006. Ants collected at Okatibbee Lake, Lauderdale County, Mississippi
- Ipser R. M. 2004. Native and exotic ants (Hymenoptera: Formicidae) of Georgia: Ecological Relationships with implications for development of biologically-based management strategies. Doctor of Philosophy thesis, University of Georgia. 165 pages.
- Ipser, R.M., M.A. Brinkman, W.A. Gardner and H.B. Peeler. 2004. A Survey of Ground-Dwelling Ants (Hymenoptera: Formicidae) in Georgia. The Florida Entomologist 87(3) 253-260.
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87.
- Ivanov K., L. Hightower, S. T. Dash, and J. B. Keiper. 2019. 150 years in the making: first comprehensive list of the ants (Hymenoptera: Formicidae) of Virginia, USA. Zootaxa 4554 (2): 532–560.
- Jeanne R. J. 1979. A latitudinal gradient in rates of ant predation. Ecology 60(6): 1211-1224.
- Johnson C. 1986. A north Florida ant fauna (Hymenoptera: Formicidae). Insecta Mundi 1: 243-246
- Kjar D. 2009. The ant community of a riparian forest in the Dyke Marsh Preserve, Fairfax County, Virginiam and a checklist of Mid-Atlantic Formicidae. Banisteria 33: 3-17.
- Kjar D., and E. M. Barrows. 2004. Arthropod community heterogeneity in a mid-Atlantic forest highly invaded by alien organisms. Banisteria 23: 26-37.
- Kjar D., and Z. Park. 2016. Increased ant (Hymenoptera: Formicidae) incidence and richness are associated with alien plant cover in a small mid-Atlantic riparian forest. Myrmecological News 22: 109-117.
- Klotz, J.H., J.R. Mangold, K.M. Vail, L.R. Davis Jr., R.S. Patterson. 1995. A survey of the urban pest ants (Hymenoptera: Formicidae) of Peninsular Florida. Florida Entomologist 78(1):109-118
- Longino, J.T. 2010. Personal Communication. Longino Collection Database
- Lubertazi, D. Personal Communication. Specimen Data from Museum of Comparative Zoology at Harvard
- Lubertazzi D. and Tschinkel WR. 2003. Ant community change across a ground vegetation gradient in north Floridas longleaf pine flatwoods. 17pp. Journal of Insect Science. 3:21
- Lynch J. F. 1988. An annotated checklist and key to the species of ants (Hymenoptera: Formicidae) of the Chesapeake Bay region. The Maryland Naturalist 31: 61-106
- MacGown J. A., J. G. Hill, R. L. Brown, T. L. Schiefer, J. G. Lewis. 2012. Ant diversity at Noxubee National Wildlife Refuge in Oktibbeha, Noxubee, and Winston Counties, Mississippi. Mississippi Agricultural and Forestry Experiment Station Bulletin 1197: 1-30
- MacGown J. A., J. G. Hill, and M. Deyrup. 2009. Ants (Hymenoptera: Formicidae) of the Little Ohoopee River Dunes, Emanuel County, Georgia. J. Entomol. Sci. 44(3): 193-197.
- MacGown J. A., J. G. Hill, and R. L. Brown. 2010. Native and exotic ant in Mississippi state parks. Proceedings: Imported Fire Ant Conference, Charleston, South Carolina, March 24-26, 2008: 74-80.
- MacGown J. A., R. L. Brown, J. G. Hill, and B. Layton. 2007. Carpenter ants of Mississippi. Mississippi Agricultural and Forestry Experiment Station Bulletin 1158: 1-35.
- MacGown J. A., and R. L. Brown. 2006. Survey of the ants (Hymenoptera: Formicidae) of the Tombigbee National Forest in Mississippi. Journal of the Kansas Entomological Society 79(4):325-340.
- MacGown J. A., and R. Whitehouse. 2015. A preliminary report of the ants of West Ship Island. A report submitted to the Gulf Islands National Seashore. Mississippi Entomological Museum Report #2015-02. 9 pp.
- MacGown, J.A and J.A. Forster. 2005. A preliminary list of the ants (Hymenoptera: Formicidae) of Alabama, U.S.A. Entomological News 116(2):61-74
- MacGown, J.A. and JV.G. Hill. Ants of the Great Smoky Mountains National Park (Tennessee and North Carolina).
- MacGown, J.A. and R.L. Brown. 2006. Survey of the ants (Hymenoptera: Formicidae) of the Tombigbee National Forest in Mississippi. Journal of the Kansas Entomological Society 79(4):325-340.
- MacGown, J.A. and T. Lockley. Ants of Horn Island, Jackson County, Mississippi
- MacGown, J.A., J.G. Hill, R.L. Brown and T.L. 2009. Ant Diversity at Noxubee National Wildlife Refuge in Oktibbeha, Noxubee, and Winston Counties, Mississippi Report #2009-01. Schiefer. 2009.
- MacGown. J. 2011. Ants collected during the 25th Annual Cross Expedition at Tims Ford State Park, Franklin County, Tennessee
- Macgown J. A., S. Y. Wang, J. G. Hill, and R. J. Whitehouse. 2017. A List of Ants (Hymenoptera: Formicidae) Collected During the 2017 William H. Cross Expedition to the Ouachita Mountains of Arkansas with New State Records. Transactions of the American Entomological Society, 143(4): 735-740.
- Mahon M. B., K. U. Campbell, and T. O. Crist. 2017. Effectiveness of Winkler litter extraction and pitfall traps in sampling ant communities and functional groups in a temperate forest. Environmental Entomology 46(3): 470–479.
- Mann H. R., E. Rowe, J. Selfridge, and D. L. Price. 2018. Leaf litter and arboreal ants (Hymenoptera: Formicidae) in a Mid-Atlantic Forest. Northeastern Naturalist 25(2): 341-354.
- O'Keefe S. T., J. L. Cook, T. Dudek, D. F. Wunneburger, M. D. Guzman, R. N. Coulson, and S. B. Vinson. 2000. The Distribution of Texas Ants. The Southwestern Entomologist 22: 1-92.
- O'Neill J.C. and Dowling A.P.G. 2011. A Survey of the Ants (hymenoptera: Formicidae) of Arkansas and the Ozark Mountains. An Undergraduate Honors, University of Arkansas. 18pages.
- Rowles, A.D. and J. Silverman. 2009. Carbohydrate supply limits invasion of natural communities by Argentine ants. Oecologia 161(1):161-171
- Smith M. R. 1935. A list of the ants of Oklahoma (Hymen.: Formicidae) (continued from page 241). Entomological News 46: 261-264.
- Smith M. R. 1962. A new species of exotic Ponera from North Carolina (Hymenoptera, Formicidae). Acta Hymenopterologica 1: 377-382.
- Sturtevant A. H. 1931. Ants collected on Cape Cod, Massachusetts. Psyche (Cambridge) 38: 73-79
- Toennisson T. A., N. J. Sanders, W. E. Klingeman, and K. M. Vail. 2011. Influences on the Structure of Suburban Ant (Hymenoptera: Formicidae) Communities and the Abundance of Tapinoma sessile. Environ. Entomol. 40(6): 1397-1404.
- Van Pelt A. F. 1948. A Preliminary Key to the Worker Ants of Alachua County, Florida. The Florida Entomologist 30(4): 57-67
- Van Pelt A. F. 1956. The ecology of the ants of the Welaka Reserve, Florida (Hymenoptera: Formicidae). American Midland Naturalist 56: 358-387
- Van Pelt A. F. 1966. Activity and density of old-field ants of the Savannah River Plant, South Carolina. Journal of the Elisha Mitchell Scientific Society 82: 35-43.
- Van Pelt A., and J. B. Gentry. 1985. The ants (Hymenoptera: Formicidae) of the Savannah River Plant, South Carolina. Dept. Energy, Savannah River Ecology Lab., Aiken, SC., Report SRO-NERP-14, 56 p.
- Warren, L.O. and E.P. Rouse. 1969. The Ants of Arkansas. Bulletin of the Agricultural Experiment Station 742:1-67
- Wetterer, James K., Espadaler, Xavier, Ashmole, M. Phillip, Mendel, Howard, Cutler, Chris and Endeman, Judith. 2007. Ants (Hymenoptera: Formicidae) of the South Atlantic Islands of Ascension Island, St. Helena, and Tristan da Cunha. Myrmecological News. 10:29-37.
- Wheeler G. C., and J. Wheeler J. 1989. A checklist of the ants of Oklahoma. Prairie Naturalist 21: 203-210.
- Wheeler W. M. 1906. Fauna of New England. 7. List of the Formicidae. Occasional Papers of the Boston Society of Natural History 7: 1-24.
- Wheeler W. M. 1910. The North American ants of the genus Camponotus Mayr. Annals of the New York Academy of Sciences 20: 295-354.
- Wheeler W. M. 1913. Ants collected in Georgia by Dr. J. C. Bradley and Mr. W. T. Davis. Psyche (Cambridge) 20: 112-117.
- Wheeler W. M. 1932. A list of the ants of Florida with descriptions of new forms. J. N. Y. Entomol. Soc. 40: 1-17.
- Whitcomb W. H., H. A. Denmark, A. P. Bhatkar, and G. L. Greene. 1972. Preliminary studies on the ants of Florida soybean fields. Florida Entomologist 55: 129-142.
- Young J., and D. E. Howell. 1964. Ants of Oklahoma. Miscellaneous Publication. Oklahoma Agricultural Experimental Station 71: 1-42.
- Young, J. and D.E. Howell. 1964. Ants of Oklahoma. Miscellaneous Publications of Oklahoma State University MP-71
- Pages using DynamicPageList3 parser function
- Photo Gallery
- Need species key
- North temperate
- North subtropical
- Cricket Associate
- Host of Myrmecophilus pergandei
- Braconid wasp Associate
- Host of Elasmosoma pergandei
- Fungus Associate
- Host of Ophiocordyceps unilateralis
- Host of Ophiocordyceps kimflemingiae
- Host of Ophiocordyceps unilateralis sensu lato
- Nematode Associate
- Host of Rabbium paradoxus
- FlightMonth
- Species
- Extant species
- Formicidae
- Formicinae
- Camponotini
- Camponotus
- Camponotus castaneus
- Formicinae species
- Camponotini species
- Camponotus species
- Ssr







