Cardiocondyla monardi
| Cardiocondyla monardi | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Crematogastrini |
| Genus: | Cardiocondyla |
| Species: | C. monardi |
| Binomial name | |
| Cardiocondyla monardi Santschi, 1930 | |
Nothing is known about the biology of Cardiocondyla monardi.
Identification
This very distinctive species should not be confused with any other African form. It is quickly separated from all its congeners in the Afrotropical region by its long scapes, lack of a metanotal groove or impression, absolutely unarmed propodeum, elongate pedicel segments and glinting silvery pubescence on a yellow background. (Bolton 1982)
Keys including this Species
Distribution
Distribution based on Regional Taxon Lists
Afrotropical Region: Angola (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
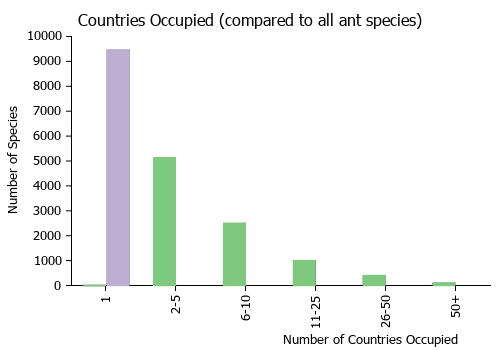
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
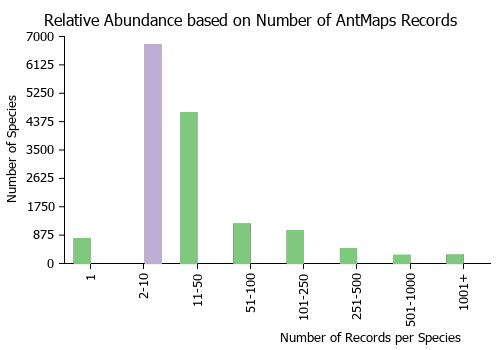
|
Biology
|
Castes
Only known from the worker caste.
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- monardi. Cardiocondyla (Loncyda) monardi Santschi, 1930b: 70, fig. 5a-c (w.) ANGOLA.
- Type-material: 2 syntype workers.
- Type-locality: Angola: Rio Mbalé, ix.1928-i.1929 (A. Monard).
- Type-depository: NHMB.
- Status as species: Bolton, 1982: 314 (redescription); Bolton, 1995b: 132; Rigato, 2002: 172 (in key).
- Distribution: Angola.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Bolton (1982) - TL 2.7, HL 0.58, HW 0.46, CI 79, SL 0.49, SI 107, PW 0.33, AL 0.68.
Antennal scapes relatively long, SI > 100; when laid back on the head exceeding the occipital corners. Maximum diameter of eye 0.14, about 0.30 x HW and with approximately 14 ommatidia in the longest row. Pronotal corners in dorsal view broadly and evenly rounded. Alitrunk in profile with the dorsum forming a single uninterrupted surface, without trace of a metanotal groove or impression. Propodeum unarmed, the dorsum rounding broadly, smoothly and evenly into the declivity. Petiole in profile with a very long anterior peduncle and a long low feebly convex node. Petiole node in dorsal view subglobular, only very slightly longer than broad. Postpetiole in dorsal view somewhat longer than broad, narrow (c. 0.13) at its junction with the petiole, then rapidly broadening posteriorly to a maximum width of c. 0.26 at about its midlength, and behind this narrowing again to a posteriormost width of c. 0.20. Dorsal length of post petiole about 0.30, of petiole peduncle plus node about 0.40. All dorsal surfaces of head, alitrunk, petiole, postpetiole and first gastral tergite reticulate-punctate. Whole of body dorsally with glinting silvery pubescence which is mostly set within the punctures. Colour yellow with glinting silvery highlights due to the pubescence.
Type Material
Angola: Rio Mbale (A. Manard).
References
- Bolton, B. 1982. Afrotropical species of the myrmecine ant genera Cardiocondyla, Leptothorax, Melissotarsus, Messor and Cataulacus (Formicidae). Bulletin of the British Museum (Natural History). Entomology, 46: 307-370 (page 314, see also)
- Santschi, F. 1930b. Résultats de la Mission scientifique suisse en Angola, 1928-1929. Formicides de l'Angola. Rev. Suisse Zool. 37: 53-81 (page 70, fig. 5 worker described)
- Seifert, B. 2022. The ant genus Cardiocondyla (Hymenoptera: Formicidae): The species groups with Oriental and Australasian origin. Diversity 15, 25 (doi:10.3390/d15010025).
References based on Global Ant Biodiversity Informatics
- Bolton B. 1982. Afrotropical species of the myrmicine ant genera Cardiocondyla, Leptothorax, Melissotarsus, Messor and Cataulacus (Formicidae). Bulletin of the British Museum (Natural History). Entomology 45: 307-370.
- Rigato F. 2002. Three new Afrotropical Cardiocondyla Emery, with a revised key to the workers (Hymenoptera Formicidae). Bollettino della Società Entomologica Italiana 134: 167-173.
- Santschi, F.. "Résultats de la Mission scientifique suisse en Angola, 1928-1929. Formicides de l'Angola." Revue Suisse de Zoologie 37 (1930): 53-81.

