Cryptopone gilva
| Cryptopone gilva | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Genus: | Cryptopone |
| Species complex: | gilva |
| Species: | C. gilva |
| Binomial name | |
| Cryptopone gilva (Roger, 1863) | |
| Synonyms | |
| |
This species occurs in a variety of mesic habitats in the southeastern United States. It nests in or under loose bark of rotten wood (Creighton and Tullock 1930). Colonies contain a few dozen to several hundred individuals and can be polygynous (Creighton and Tullock 1930; Haskins 1931; Smith 1934, 1944). Haskins (1931) provided an account of the behavior of a captive colony, with details of brood development and worker behavior. (Branstetter & Longino, 2022)
| At a Glance | • Polygynous |
Identification
From Mackay and Mackay (2010): The conical setae on the middle tibia would separate workers and females of C. gilva from most of the others in the genus. This species could be most easily confused with Pseudoponera stigma based on the number of mandibular teeth and the shape of the subpetiolar process. Cryptopone gilva can be easily separated as the medial part of the clypeus is not pinched in P. stigma, as it is in C. gilva. The worker and female are easily separated from the other members of the ochracea species complex. They are dull, not shining like the worker of Centromyrmex brachycola and it has 7 mandibular teeth, usually not 5 (reduced to 4 in some specimens) as in Wadeura guianensis. The worker of C. gilva is nearly identical to that of Wadeura holmgreni. It can be distinguished by lacking a tooth on the medial border of the clypeus, having a slightly depressed dorsal face of the propodeum, as compared to the level of the mesonotum and in having a translucent anterior half of the subpetiolar process.
The worker of C. gilva is easily confused with members of the genus Hypoponera. The mandibles of C. gilva are similar to those in the Pseudoponera stigma species complex (with distinct teeth and not with a series of small teeth or denticles as is common in Hypoponera), but otherwise many of the other characteristics of C. gilva could show a close resemblance to Hypoponera. The medial part of the clypeus of C. gilva is pinched laterally, similar to that in some of the Neotropical species of Hypoponera. The mesosoma, petiole and subpetiolar process of C. gilva are similar to those in the genus Hypoponera. It is difficult to see the smallest tibial spurs on the middle and posterior tibiae of C. gilva.
Keys including this Species
Distribution
Southern United States through Central America. (Mackay and Mackay 2010)
Latitudinal Distribution Pattern
Latitudinal Range: 36.24013889° to 27°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps; Branstetter & Longino, 2022
Distribution based on Regional Taxon Lists
Nearctic Region: United States (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
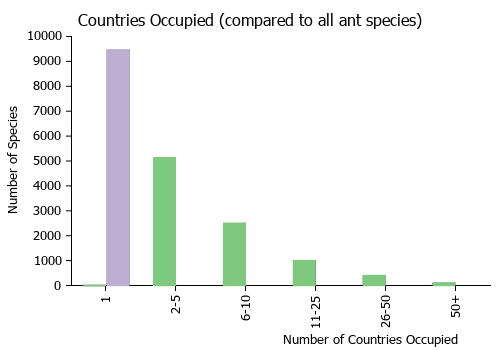
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Habitat
Cryptopone gilva is a common ant found in forests and disturbed urban sites, tropical rain forests and cloud forests, from sea level up to 2000 meters elevation. (Mackay and Mackay 2010)
Biology
Mackay and Mackay (2010) -This species nests in rotten logs or under bark on the forest floor in areas with rocky loam soils. In the United States they are found in woody frass just beneath the bark of pine logs and stumps (Smith, 1934). It is usually in logs that are still solid but with loose bark and with a thin layer of decayed humus between the bark and the wood (Longino, 2006). Often the log is clear of the ground for at least part of its length on the under side of the log (Creighton and Tulloch, 1930). Longino (2006) reported it occurring under epiphytes mats in the low arboreal zone. Colonies are small with a few dozen to about 100 individuals (Smith, 1944). Males and winged females are generally produced in May and June in Florida (Smith, 1944). Brood and females were present in a nest in Nicaragua in July and sexuals were in a nest in November (Florida). Sexuals are produced during May and June in Mississippi. A nuptial flight probably occurs (Haskins, 1931). Longino (2006) mentions that lone founding queens are common. Nests include one to several hundred workers and often as many as 10 dealate queens (Haskins, 1931; Smith, 1934). The ants are slow and feign death (Smith, 1929). The males are very active and difficult to capture. Specimens can be extracted in Winkler samples (Longino, 2006).
Workers are predaceous (Maes, 1989) but accept meat, fruit and honey in artificial nests (Haskins, 1931).
Haskins (1931) provided detailed notes on the behavior and biology of an artificial colony of this species. Among the many observations he noted that eggs hatch after about 30 days (23°C). The newly hatched larvae are immediately moved to a separate chamber away from the eggs (to avoid cannibalism?) and are fed pieces of solid food. The workers lick the secretions from the larvae and bite the larvae apparently to hasten the flow of exudates. The larval stage requires about 25 days. The workers cover the mature larvae with soil and after the larvae spin a cocoon (19 hours) they are uncovered and cleaned. The pupal stage of workers requires about 32 days and a male 36 days. If a larva is not covered with soil it transforms into a semipupa and is then usually destroyed by the workers. The cocoon is surrounded by workers during eclosion (½ hour), which bite and tear the anterior end, but usually the callow actually opens the cocoon. The workers lick and pinch the callow with their mandibles and treat them similar to the larvae. The callows remain helpless for a day and then participate on the duties inside the nest. After this period they forage when they are still light in color (for up to 21 days). The workers cannot see well, but will place the brood under an area of red light (occasionally blue or green) when exposed to a spectrum. Workers and females respond aggressively to sudden sounds.
Longino (2006) - I have found Cryptopone to be relatively common in Costa Rican cloud forest habitats. For example, it is common in the ridge crest cloud forest in the Monteverde area (1400 - 1600 m), rare around “El Aleman” at the head of the Penas Blancas Valley (900 m), and absent at Casa Eladio further down the valley (800 m). On the Barva Transect in Braulio Carrillo National Park, it occurs in a narrow elevational band from 1000 - 1500 m. It is common under loose bark of dead wood and under epiphyte mats in the low arboreal zone: ground level to a few meters high. I often encounter lone founding queens. I find colonies in logs at a certain stage of decay, when the bark comes off in intact sheets, and there is a thin layer of decayed humus between the bark and the still hard wood. Workers are found thinly scattered in anastomosing tunnels in the humus layer. As a bark sheet is peeled away one to five workers may be revealed, which quickly disappear into holes in the wood and under adjacent bark. I have never been able to collect more than a few dozen workers from a colony, and I have never found an obvious colony center or distinct galleries with aggregations of workers and brood. Occasional larvae and pupae occur in the tunnels. The nesting behavior is very similar to that of Typhlomyrmex rogenhoferi, a species more common at lower elevations. Specimens are occasionally taken in samples of sifted leaf litter (Winkler samples).
Castes
Worker
Images from AntWeb
   
| |
| Worker. Specimen code casent0006007. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Worker. Specimen code casent0006054. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Worker. Specimen code casent0103829. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
Queen
Images from AntWeb
   
| |
| Queen (alate/dealate). Specimen code casent0006055. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Queen (alate/dealate). Specimen code casent0006056. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
    
| |
| Queen (alate/dealate). Specimen code casent0104163. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
Male
Images from AntWeb
   
| |
| Male (alate). Specimen code casent0103825. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
Phylogeny
Placement of this species within the subfamily Ponerinae
| Ponerinae |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See Phylogeny of Ponerinae for details.
| C. gilva complex |
| ||||||||||||||||||
Based on Branstetter & Longino (2022).
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- gilva. Ponera gilva Roger, 1863a: 170 (w.) U.S.A. (no state data).
- Type-material: syntype workers (number not stated).
- Type-locality: North America (no further data).
- Type-depository: MNHU.
- Creighton & Tulloch, 1930: 74 (q.m.); Wheeler, G.C. & Wheeler, J. 1952c: 625 (l.).
- Combination in Pachycondyla (Pseudoponera): Emery, 1901a: 46;
- combination in Euponera (Trachymesopus): Emery, 1911d: 86;
- combination in Trachymesopus: Kempf, 1960f: 424;
- combination in Pachycondyla: Mackay & Mackay, 2010: 352;
- combination in Cryptopone: Brown, 1963: 3; Schmidt, C.A. & Shattuck, 2014: 185.
- Status as species: Roger, 1863b: 16; Mayr, 1863: 448; Mayr, 1886d: 438; Cresson, 1887: 258; Dalla Torre, 1893: 39; Emery, 1895c: 266; Emery, 1896g: 54 (in key); Wheeler, W.M. 1910g: 561; Emery, 1911d: 86; Smith, M.R. 1928b: 244; Creighton & Tulloch, 1930: 74; Dennis, 1938: 277; Smith, M.R. 1944d: 14; Creighton, 1950a: 46; Smith, M.R. 1951a: 786; Smith, M.R. 1958c: 111; Carter, 1962a: 6 (in list); Smith, M.R. 1967: 348; Smith, D.R. 1979: 1341; Deyrup, et al. 1989: 93; Bolton, 1995b: 166; Deyrup, 2003: 44; MacGown & Forster, 2005: 67; Longino, 2006b: 135; Mackay & Mackay, 2010: 352 (redescription); Deyrup, 2017: 20 Fernandes & Delabie, 2019: 411 (in key); Branstetter & Longino, 2022: 15.
- Senior synonym of harnedi: Creighton & Tulloch, 1930: 74; Creighton, 1950a: 46; Smith, M.R. 1951a: 786; Smith, D.R. 1979: 1341; Bolton, 1995b: 166; Longino, 2006b: 135; Mackay & Mackay, 2010: 352; Branstetter & Longino, 2022: 15.
- Distribution: U.S.A.
- harnedi. Euponera (Trachymesopus) gilva subsp. harnedi Smith, M.R. 1929: 543 (w.) U.S.A. (Mississippi).
- Type-material: syntype workers (number not stated, “numerous”).
- Type-locality: U.S.A.: Mississippi, Columbus (M.R. Smith).
- Type-depositories: MCZC, MSUS, USNM.
- Junior synonym of gilva: Creighton & Tulloch, 1930: 74; Creighton, 1950a: 46; Smith, M.R. 1951a: 786; Smith, D.R. 1979: 1341; Bolton, 1995b: 166; Longino, 2006b: 135; Mackay & Mackay, 2010: 352; Branstetter & Longino, 2022: 15.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
From Mackay and Mackay (2010): The worker is a small (total length 2.8 - 4 mm) ferruginous red to yellowish brown ant with tiny eyes (maximum diameter 0.04 mm, may be absent). The mandible is elongate and shining, and has at least five teeth (usually 6), which are approximately the same size (the apical tooth is slightly larger and a small bump may be present on the basal mandibular border). The anterior border of the clypeus is broadly convex, the central part of the clypeus has a swollen region, which nearly forms a carina and is surrounded by concave areas, which give it the appearance of having been “pinched” from the two sides. The distance between the anterior border of the eye and the border of the head is at least three times the maximum eye length (side view). The antennal scapes are relatively short and fail to reach the posterior lateral corner by one to three funicular segments. The dorsum of the mesosoma is broadly rounded or somewhat flattened. The pronotal shoulder is only slightly swollen, the mesosoma is moderately depressed at the metanotal suture and the dorsal face of propodeum is nearly straight, meeting the posterior face at an angle.
The anterior part of the side of the propodeum is deeply impressed, apparently for the reception of the middle femur. The propodeal spiracle is circular. The petiole is somewhat thickened when viewed in profile and is narrowed toward the apex, but still somewhat rectangular shaped. The subpetiolar process is broadly rounded anteriorly, developed into a translucent region anteriorly and a broad thickened lobe posteriorly. The lobe forms a sharp edge when viewed from below. The dorsum of the gaster is without a stridulatory file. Most surfaces are dull and punctate, the top of the mesosoma, the pronotum, the mesopleuron, the side of the propodeum and petiole and the dorsum of the gaster are weakly shining.
Erect hairs are scattered, but sparse on the mandibles, clypeus, the dorsal and ventral surfaces of the head, on the scapes, on the dorsum of the mesosoma, dorsum of the petiole and all surfaces of the gaster. Appressed golden or silver erect pubescence (0.02 mm) is abundant on most surfaces. The tibia of the middle leg has a number of very coarse hairs (conical setae) on the extensor surface, which are much thicker than other hairs and are lacking on the anterior and the posterior tibiae.
Queen
From Mackay and Mackay (2010): The female is similar to the worker except larger (total length slightly over 4 mm) and has larger eyes (maximum diameter 0.17 mm), ocelli and the mesosoma is winged and adapted for flight. The mandibles, clypeus and petiole are similar to those of the worker. The translucent area is also present on the subpetiolar process.
The pilosity and sculpture are similar to that of the worker. The conical setae on the middle tibia are slightly more developed than in the worker.
Male
From Mackay and Mackay (2010): The male is a small (total length about 4 mm) ant. The male has a 13-segmented antenna in which the antennae (3.5 mm) are nearly as long as the remainder of the ant. The mandibles are tiny edentate and fail to touch by distance equal to at least their length. They have depressions near the bases. The eyes are relatively small but occupy about ½ of the side of the head. The scape is short, about twice the length of the first funicular segment. The mesosoma is also enlarged. The parapsidal sutures are present but the Mayrian furrows are not developed. The petiole is small and rounded dorsally; the subpetiolar process is little developed. The middle tibia does not have the conical setae that are found in the worker and female. The translucent area is not present on the subpetiolar process.
Short (mostly less than 0.02 mm in length) erect bristly hairs are present on nearly all surfaces including the eyes. Most surfaces are punctate and weakly shining; the side of the petiole is slightly glossy.
Type Material
North America (without specific locality) (Mackay and Mackay 2010)
Etymology
The species name is from the Latin word gilvus, meaning pale yellow. Creighton and Tulloch (1930) pointed out the name is a misnomer as the specimens are dark brown. (Mackay and Mackay 2010)
References
- Alatorre-Bracamontes, C.E., Vásquez-Bolaños, M. 2010. Lista comentada de las hormigas (Hymenoptera: Formicidae) del norte de México. Dugesiana 17(1): 9-36.
- Branstetter, M.G., Longino, J.T. 2022. UCE phylogenomics of New World Cryptopone (Hymenoptera: Formicidae) elucidates genus boundaries, species boundaries, and the vicariant history of a temperate–tropical disjunction. Insect Systematics & Diversity 6(1): 6:1-23 (doi:10.1093/isd/ixab031).
- Creighton, W. S.; Tulloch, G. S. 1930. Notes on Euponera gilva (Roger) (Hymenoptera, Formicidae). Psyche (Camb.) 37: 71-79.
- Esteves, F.A., Fisher, B.L. 2021. Corrieopone nouragues gen. nov., sp. nov., a new Ponerinae from French Guiana (Hymenoptera, Formicidae). ZooKeys 1074, 83–173 (doi:10.3897/zookeys.1074.75551).
- Fernandes, I.O., Delabie, J.H.C. 2019. A new species of Cryptopone Emery (Hymenoptera: Formicidae: Ponerinae) from Brazil with observations on the genus and a key for New Word species. Sociobiology 66: 408-441 (doi:10.13102/sociobiology.v66i3.4354).
- Haskins, C. 1931. Notes on the Biology and social life of Euponera gilva Roger var. harnedi M. R. Smith. Journal of the New York Entomological Society 39:507-521.
- Ipser, R.M., Brinkman, M.A., Gardner, W.A., Peeler, H.B. 2004. A survey of ground-dwelling ants (Hymenoptera: Formicidae) in Georgia. Florida Entomologist 87: 253-260.
- Longino, J. T. 2006. New species and nomenclatural changes for the Costa Rican ant fauna (Hymenoptera: Formicidae). Myrmecologische Nachrichten 8:131-143.
- Mackay, W.P., Mackay, E.E. 2010. The systematics and biology of the New World ants of the genus Pachycondyla (Hymenoptera: Formicidae). Edwin Mellon Press, Lewiston.
- Maes, J.-M. 1989. Catálogo de los insectos controladores Biológicas en Nicaragua. Volumen I. Insectos depredadores (Primera parte). Revista Nicaraguense de Entomología 8:1-106.
- Richter, A., Boudinot, B.E., Hita Garcia, F., Billen, J., Economo, E.P., Beutel, R.G. 2023. Wonderfully weird: the head anatomy of the armadillo ant, Tatuidris tatusia (Hymenoptera: Formicidae: Agroecomyrmecinae), with evolutionary implications. Myrmecological News 33: 35-75 (doi:10.25849/MYRMECOL.NEWS_033:035).
- Roger, J. 1863b. Die neu aufgeführten Gattungen und Arten meines Formiciden -Verzeichnisses, nebst Ergänzung einiger früher gegeben-en Beschreibungen. Berliner Entomologische Zeitschrift 7:131-214.
- Schmidt, C.A. & Shattuck, S.O. 2014. The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of ponerine ecology and behavior. Zootaxa 3817, 1–242 (doi:10.11646/zootaxa.3817.1.1).
- Smith, M. R. 1929. Descriptions of five new North American ants, with biological notes. Annals of the Entomological Society of America 22:543-551.
- Smith, M. R. 1934. Ponerine ants of the genus Euponera in the United States. Annals of the Entomological Society of America 27:557-564.
- Smith, M. R. 1944. Additional ants recorded from Florida, with descriptions of two new subspecies. The Florida Entomologist 27:14-17.
References based on Global Ant Biodiversity Informatics
- Alatorre-Bracamontes, C.E. and M Vasquez-Bolanos. 2010. Lista comentada de las hormigas (Hymenoptera: Formicidae) del norte de México. Dugesiana 17(1):9-36
- Brown W. L., Jr. 1974. A remarkable new island isolate in the genus Proceratium (Hymenoptera: Formicidae). Psyche (Camb.) 81: 70-83.
- CSIRO Collection
- Creighton W. S., and G. S. Tulloch. 1930. Notes on Euponera gilva (Roger) (Hymenoptera, Formicidae). Psyche (Camb.) 37: 71-79.
- Dash S. T. and L. M. Hooper-Bui. 2008. Species diversity of ants (Hymenoptera: Formicidae) in Louisiana. Conservation Biology and Biodiversity. 101: 1056-1066
- Dattilo W. et al. 2019. MEXICO ANTS: incidence and abundance along the Nearctic-Neotropical interface. Ecology https://doi.org/10.1002/ecy.2944
- Deyrup, M. 2003. An updated list of Florida ants (Hymenoptera: Formicidae). Florida Entomologist 86(1):43-48.
- Fernández F., and T. M. Arias-Penna. 2008. Las hormigas cazadoras en la región Neotropical. Pp. 3-39 in: Jiménez, E.; Fernández, F.; Arias, T.M.; Lozano-Zambrano, F. H. (eds.) 2008. Sistemática, biogeografía y conservación de las hormigas cazadoras de Colombia. Bogotá: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, xiv + 609 pp.
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Forster J.A. 2005. The Ants (hymenoptera: Formicidae) of Alabama. Master of Science, Auburn University. 242 pages.
- General D., and L. Thompson. 2008. New distributional records of ants in Arkansas. Journal of the Arkansas Academy of Science 62: 148-150.
- Guénard B., K. A. Mccaffrey, A. Lucky, and R. R. Dunn. 2012. Ants of North Carolina: an updated list (Hymenoptera: Formicidae). Zootaxa 3552: 1-36.
- INBio Collection (via Gbif)
- Johnson C. 1986. A north Florida ant fauna (Hymenoptera: Formicidae). Insecta Mundi 1: 243-246
- Kempf W. W. 1960. Miscellaneous studies on Neotropical ants (Hymenoptera, Formicidae). Studia Entomologica (n.s.)3: 417-466.
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Longino J. T. 2006. New species and nomenclatural changes for the Costa Rican ant fauna (Hymenoptera: Formicidae). Myrmecologische Nachrichten 8: 131-143.
- Longino J. T. L., and M. G. Branstetter. 2018. The truncated bell: an enigmatic but pervasive elevational diversity pattern in Middle American ants. Ecography 41: 1-12.
- Longino J. T., and R. K. Colwell. 2011. Density compensation, species composition, and richness of ants on a neotropical elevational gradient. Ecosphere 2(3): 16pp.
- Longino J. et al. ADMAC project. Accessed on March 24th 2017 at https://sites.google.com/site/admacsite/
- Longino, J.T. 2010. Personal Communication. Longino Collection Database
- MacGown J. A., J. G. Hill, R. L. Brown, T. L. Schiefer, J. G. Lewis. 2012. Ant diversity at Noxubee National Wildlife Refuge in Oktibbeha, Noxubee, and Winston Counties, Mississippi. Mississippi Agricultural and Forestry Experiment Station Bulletin 1197: 1-30
- MacGown J. A., J. G. Hill, and M. Deyrup. 2009. Ants (Hymenoptera: Formicidae) of the Little Ohoopee River Dunes, Emanuel County, Georgia. J. Entomol. Sci. 44(3): 193-197.
- MacGown J. A., J. G. Hill, and R. L. Brown. 2010. Native and exotic ant in Mississippi state parks. Proceedings: Imported Fire Ant Conference, Charleston, South Carolina, March 24-26, 2008: 74-80.
- MacGown J. A., and R. L. Brown. 2006. Survey of the ants (Hymenoptera: Formicidae) of the Tombigbee National Forest in Mississippi. Journal of the Kansas Entomological Society 79(4):325-340.
- MacGown, J.A and J.A. Forster. 2005. A preliminary list of the ants (Hymenoptera: Formicidae) of Alabama, U.S.A. Entomological News 116(2):61-74
- MacGown, J.A. and JV.G. Hill. Ants of the Great Smoky Mountains National Park (Tennessee and North Carolina).
- MacGown, J.A. and R.L. Brown. 2006. Survey of the ants (Hymenoptera: Formicidae) of the Tombigbee National Forest in Mississippi. Journal of the Kansas Entomological Society 79(4):325-340.
- MacGown, J.A., J.G. Hill, R.L. Brown and T.L. 2009. Ant Diversity at Noxubee National Wildlife Refuge in Oktibbeha, Noxubee, and Winston Counties, Mississippi Report #2009-01. Schiefer. 2009.
- MacGown. J. 2011. Ants collected during the 25th Annual Cross Expedition at Tims Ford State Park, Franklin County, Tennessee
- Mackay, W.P. and E.E. MacKay. 2010. The systematics and biology of the New World ants of the genus Pachycondyla (Hymenoptera: Formicidae). Edwin Mellen Press Lewiston, NY
- Maes, J.-M. and W.P. MacKay. 1993. Catalogo de las hormigas (Hymenoptera: Formicidae) de Nicaragua. Revista Nicaraguense de Entomologia 23.
- Menozzi C. 1931. Qualche nuova formica di Costa Rica (Hym.). Stettiner Entomologische Zeitung. 92: 188-202.
- O'Keefe S. T., J. L. Cook, T. Dudek, D. F. Wunneburger, M. D. Guzman, R. N. Coulson, and S. B. Vinson. 2000. The Distribution of Texas Ants. The Southwestern Entomologist 22: 1-92.
- Smith M. A., W. Hallwachs, D. H. Janzen. 2014. Diversity and phylogenetic community structure of ants along a Costa Rican elevational gradient. Ecography 37(8): 720-731.
- Van Pelt A. F. 1948. A Preliminary Key to the Worker Ants of Alachua County, Florida. The Florida Entomologist 30(4): 57-67
- Van Pelt A. F. 1956. The ecology of the ants of the Welaka Reserve, Florida (Hymenoptera: Formicidae). American Midland Naturalist 56: 358-387
- Van Pelt A. F. 1966. Activity and density of old-field ants of the Savannah River Plant, South Carolina. Journal of the Elisha Mitchell Scientific Society 82: 35-43.
- Van Pelt A., and J. B. Gentry. 1985. The ants (Hymenoptera: Formicidae) of the Savannah River Plant, South Carolina. Dept. Energy, Savannah River Ecology Lab., Aiken, SC., Report SRO-NERP-14, 56 p.
- Vásquez-Bolaños M. 2011. Lista de especies de hormigas (Hymenoptera: Formicidae) para México. Dugesiana 18: 95-133
- Weber N. A. 1943. The ants of the Imatong Mountains, Anglo-Egyptian Sudan. Bulletin of the Museum of Comparative Zoology 93: 263-389.
- Wheeler, G.C. and J. Wheeler. 1985. A checklist of Texas ants. Prairie Naturalist 17:49-64.

