Neoponera goeldii
| Neoponera goeldii | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Genus: | Neoponera |
| Species: | N. goeldii |
| Binomial name | |
| Neoponera goeldii Forel, 1912 | |
| Synonyms | |
| |
One of the few arboreal species of Ponerinae, this generalist predator is characterized by the agility and swiftness of its capture behaviour (Orivel et al. 2000). Foraging is nocturnal, potential prey are diurnal insects sheltered for the night under leaves. Foragers also gather and retrieve extrafloral nectar. They are known to form ant gardens.
| At a Glance | • Ant garden |
Identification
From Mackay and Mackay (2010): The shape of the petiole of the worker of N. goeldii would separate it from most of the others in the crenata species complex (anterior and posterior faces are convex with the highest point near the middle of the apex). Only four species have a petiole with a similar shape: Neoponera cavinodis, Neoponera donosoi, Neoponera oberthueri and Neoponera carinulata. Neoponera cavinodis and N. oberthueri are easily separated from N. goeldii as the highest point is closer to the posterior end the dorsum of the petiole and the posterior face of the petiole is weakly to strongly concave. The anterior face of the petiole of N. goeldii forms a continuously sloping curve and is not angulate about half way up the node as it is N. carinulata.
The workers of N. goeldii are similar to those of N. donosoi. They can be separated on the basis of three well-defined characters: the dorsal surfaces of the mandibles of N. goeldii are completely striate, whereas they are mostly smooth and glossy in N. donosoi. The subpetiolar process is strongly concave in N. goeldii, not weakly concave as in N. donosoi and the tibiae of N. goeldii have abundant erect hairs that are at least as long as the diameter tibia, often twice or more as long as compared with the sparse hairs in N. donosoi in which the hairs are approximately as long as the diameter of the tibiae. The female of N. goeldii could be easily confused with that of Neoponera unidentata, but the long hairs on the middle and posterior tibiae (most longer than the diameter of the tibiae) would separate it from N. unidentata, in which the length of these hairs is approximately equal to the diameter of the tibiae.
It would not be possible to confuse males of N. goeldii with those of N. donosoi. The males of N. goeldii are very distinct in that they are about as large as the workers, not approximately ½ the size the workers as in N. donosoi. The subpetiolar process is strongly concave in N. goeldii, but barely can be described as concave in males of N. donosoi.
Santschi (1920) states that Neoponera lydiae differs from N. goeldii in lacking the dorsal face of the petiole. Comparison of females associated with workers of N. goeldii, with the lectotype of N. lydiae, shows them to be identical. Both forms have the extremely long erect hairs on the scapes and tibiae.
Distribution
ECUADOR, GUYANA, FRENCH GUIANA, SURINAME, PERU, TRINIDAD, BRASIL (Mackay and Mackay 2010)
Latitudinal Distribution Pattern
Latitudinal Range: 10.68° to -12.043°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Brazil (type locality), French Guiana.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
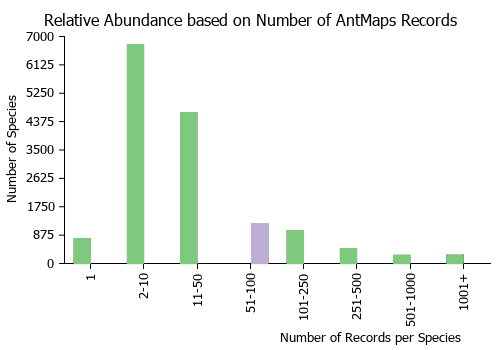
|
Habitats
Neoponera goeldii is found in a number of habitats ranging from caatinga (scrub vegetation), riparian rain forest, wet forest, second growth rain forest, a clearing in second growth rain forest and in an urban habitat (garden), at elevations ranging from 250 - 770 m. Nests are located in open sunny areas (Belin-Depoux, 1991, Mackay and Mackay 2010).
Biology
From Mackay and Mackay (2010): Nests are usually found in hollow twigs or branches (1 - 7 cm in diameter), often those found on the forest floor. It also constructs carton nests (Corbara and Dejean, 1996; Orivel, et al., 1998). The nest described by Weber (1944) in Trinidad is probably that of N. goeldii. The specimens from near Oropuche Cave were on a pomerac [Syzygium] tree at a roadside. Brood and a male were collected in a nest in July (Ecuador). Alate females were collected in May (Ecuador and Suriname); alate males were collected in October and November (Perú). Dealate females were collected in April (French Guiana), June (Trinidad) and July (Perú). Workers are extremely fast and difficult to capture.
The specimens from near Manaus were in an epiphyte (Anthurium cf. gracile [Araceae]). The ants integrate epiphyte seeds in their nests (Aechmea mertensii [Bromeliaceae], Clusia sp. [Clusiaceae], Codonanthe calcarata [Gesneriaceae], Peperomia macrostachya [Piperaceae] and Anthurium gracile [Araceae]), which germinate, develop and reinforce the nest (Orivel et al., 1998).
Caterpillars of Vettius tertianus (Hesperiidae) live in the nests in the bromeliad Aechmea mertensii. Neoponera goeldii nests together with the ponerine ant Odontomachus mayi (Corbara et al., 1999).
Ant Gardens
This species is known to form ant gardens (i.e., they are able to initiate ant gardens or are restricted to ant gardens) (Benson, 1985; Campbell et al., 2022; Corbara & Dejean, 1996; Dejean et al., 2000; Orivel & Leroy, 2011; Orivel et al., 1997; Orivel et al., 1998 (noted as ant-garden initiator); Orivel et al., 1999 (noted as ant-garden initiator).
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
This species is a host for the encyrtid wasp Blanchardiscus pollux (a parasite) (Universal Chalcidoidea Database) (primary host).
Castes
Worker
Images from AntWeb
    
| |
| Lectotype of Pachycondyla goeldii. Worker. Specimen code casent0907240. Photographer Z. Lieberman, uploaded by California Academy of Sciences. | Owned by MHNG, Geneva, Switzerland. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- goeldii. Neoponera goeldii Forel, 1912c: 36 (w.) BRAZIL (Amazonas).
- Type-material: lectotype worker (by designation of Mackay & Mackay, 2010: 362), 1 paralectotype worker.
- Type-locality: lectotype Brazil: Amazonas, Victoria (Goeldi); paralectotype with same data.
- Type-depository: MHNG.
- Mackay & Mackay, 2010: 363 (m.).
- Combination in Pachycondyla: Brown, in Bolton, 1995b: 305;
- combination in Neoponera: Schmidt, C.A. & Shattuck, 2014: 151.
- Status as species: Borgmeier, 1923: 66; Kempf, 1972a: 162; Bolton, 1995b: 305; Mackay, Mackay, et al. 2008: 192; Mackay & Mackay, 2010: 362 (redescription); Bezděčková, et al. 2015: 123; Feitosa, 2015c: 99; Fernández & Guerrero, 2019: 534.
- Senior synonym of lydiae: Mackay & Mackay, 2010: 362.
- Distribution: Brazil, Ecuador, French Guiana, Guyana, Peru, Suriname, Trinidad.
- lydiae. Neoponera lydiae Santschi, 1920d: 361 (q.) FRENCH GUIANA.
- Type-material: holotype queen.
- Type-locality: French Guiana: Nouveaux Chantiers, vii (Le Moult).
- Type-depository: NHMB.
- Combination in Pachycondyla: Brown, in Bolton, 1995b: 307.
- Status as species: Kempf, 1972a: 162; Bolton, 1995b: 307.
- Junior synonym of goeldii: Mackay & Mackay, 2010: 362.
Type Material
Brasil: Amazonas: Vitória; French Guiana: Nouveaux Chantiers. 1 lectotype, 1 paralectotype here designated, Musee d'Histoire Naturelle Genève; holotype seen, Naturhistorisches Museum, Basel (Mackay and Mackay 2010)
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
From Mackay and Mackay (2010): The worker is a moderate sized (total length 7 - 8 millimeters) yellowish brown to brown ant. The mandibles are moderately elongated and have about 13 teeth. The anterior medial border of the clypeus is angulate. The sides of the head are nearly parallel but noticeably narrowed anterior to the eyes. The eyes are relatively large, occupying about ⅓ of the side of the head. The malar carina is well developed and extends to the anterior margin of the eye. The scape is long and extends about three funicular segments past the posterior lateral corner of the head. The posterior margin is nearly straight. The pronotal shoulder is formed into a sharp carina which slightly overhangs the side of the propodeum. The metanotal suture is barely marked on the dorsum of the mesosoma and is only slightly depressed below the outline. The propodeal spiracle is elongated. Both the anterior and posterior faces of the petiole are convex (at least the upper half of the anterior face) and meet at the highest point in the middle of the apex. The subpetiolar process is poorly developed. The anterior face of the postpetiole is slightly concave and meets the broadly rounded dorsal face at slightly more than a 90 degree angle. The metasternal process consists of two closely spaced lobes with the surface between them crenulated.
Erect hairs are abundant and long on most surfaces, including the mandibles, the clypeus (up to 0.6 mm), on the scapes (0.3 mm, length > diameter of scape), dorsum of the mesosoma (up to 0.5 mm), petiole and gaster, the hairs on the legs are long, especially those on the middle and posterior tibiae (up to 0.4 mm), most longer than the diameter of the tibiae; appressed silver hairs are scattered on most surfaces and moderately dense on the dorsum of the gaster.
The mandibles are finely striate and dull, the clypeus is depressed medially with poorly developed striate on both sides of the depression. The head, mesosoma, petiole and gaster are covered with poorly defined punctures and moderately smooth and glossy.
Queen
From Mackay and Mackay (2010): The female (Neoponera lydiae) is similar in most aspects, including the presence of long hairs, mostly longer than the diameter of the appendages, differing from the worker only significantly in the shape of the petiole, in which the anterior face of the petiole is nearly straight and the posterior face is broadly rounded and meets near the anterior edge of the apex.
The pronotum has a sharp carina on the shoulder and the propodeal spiracle is slit-shaped.
The pilosity and sculpture are similar to that of the worker.
Male
From Mackay and Mackay (2010): The male (undescribed) is a small (total length 6 mm) dark brown specimen with yellowish brown appendages, clypeus and genitalia. The head length is 0.96 mm; head width 0.79 mm. The eye is large (maximum diameter 0.60 mm) located less then ½ diameter from the lateral ocellus. The median ocellus (0.13 mm) is located approximately one diameter from the lateral ocellus (0.14 mm). The pronotal shoulder is only slightly swollen; the Mayrian furrows are well developed on the scutum, as are the parapsidal sutures. The scutellum is only weakly convex. The propodeal spiracle is slit-shaped. The petiole is triangular-shaped with the anterior and posterior faces being very similar shaped. The subpetiolar process consists of a rounded anterior lobe followed by a concave region and a small angle. The anterior face of the postpetiole is broadly rounded; the subpostpetiolar process is poorly developed. The specimen is a callow and the wing venation is difficult to see. The genitalia were not dissected on the single available specimen.
Erect hairs are sparse and range from 0.1 - 0.2 mm in length. These hairs are present on the clypeus, dorsal and ventral surfaces of the head, posterior margin of the head, mesosoma, petiole and subpetiolar process and all surfaces of the gaster. The legs, including the tibiae, have similar erect and suberect hairs. Erect hairs on the tibiae are approximately as long as the diameter of the tibiae.
The head is finely sculptured and moderately shining, the sculpture on the mesosoma is similar and most surfaces are shining and nearly smooth and glossy (the mesopleuron), the side of the propodeum has poorly defined rugae. The petiole has a single longitudinal ruga; the gaster is mostly smooth and shining.
Karyotype
- n = 12, 2n = 24, karyotype = 24A (French Guiana) (Mariano et al., 2007; Mariano et al., 2011; Mariano et al., 2015) (as Pachycondyla goeldii).
Etymology
This species was named in honor of Mr. Göldi who collected the type series. (Mackay and Mackay 2010)
References
- Benson, W.W. 1985. Amazon ant-plants. In: Prance, G.T. & Lovejoy, T.E. (Eds.): Amazonia. Pergamon Press, Oxford, pp. 239-266.
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Araujo, E.S., Koch, E.B.A., Delabie, J.H.C., Zeppelini, D., DaRocha, W.D., Castaño-Meneses, G., Mariano, C.S.F. 2019. Diversity of commensals within nests of ants of the genus Neoponera (Hymenoptera: Formicidae: Ponerinae) in Bahia, Brazil. Annales de la Société entomologique de France (N.S.) 55, 291–299 (doi:10.1080/00379271.2019.1629837).
- Barassé, V., Touchard, A., Téné, N., Tindo, M., Kenne, M., Klopp, C., Dejean, A., Bonnafé, E., Treilhou, M. 2019. The peptide venom composition of the fierce stinging ant Tetraponera aethiops (Formicidae: Pseudomyrmecinae). Toxins 11, 732 (doi:10.3390/toxins11120732).
- Belin-Depoux, M. 1991. Écologie évolutive des jardins de fourmis en Guyane française. Rev. Ecol. Terre Vie 46:1-38.
- Brown, W. L., Jr. 1995a. [Untitled. Taxonomic changes in Pachycondyla attributed to Brown.] Pp. 302-311 in: Bolton, B. A new general catalogue of the ants of the world. Cambridge, Mass.: Harvard University Press, 504 pp. (page 305, Combination in Pachycondyla)
- Campbell, L.C.E., Kiers, E.T., Chomicki, G. 2022. The evolution of plant cultivation by ants. Trends in Plant Science (doi:10.1016/j.tplants.2022.09.005).
- Corbara, B., Dejean, A. 1996. Arboreal nest building and ant-garden initiation by a Ponerine ant. Naturwissenschaften 83, 227–230 (doi:10.1007/BF01143330).
- Corbara, B., A. Dejean and J. Orivel. 1999. Ant gardens, a unique epiphyte-ant association. L’Année Biologique 38:73-89.
- Dejean, A., Corbara, B., Orivel, J., Snelling, R.R., Delabie, J.H.C., Belin-Depoux, M. 2000. The importance of ant gardens in the pioneer vegetal formations of French Guiana. Sociobiology 35: 425-439.
- Forel, A. 1912. Formicides Néotropiques. Part 1. Annales de la Société Entomologique de Belgique 56:28-49.
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- Mackay, W.P., Mackay, E.E. 2010. The systematics and biology of the New World ants of the genus Pachycondyla (Hymenoptera: Formicidae). Edwin Mellon Press, Lewiston.
- Mariano, C. d. S. F., Pompolo, S. d. G., Silva, J. G. & Delabie, J. H. C. 2011. Contribution of cytogenetics to the debate on the paraphyly of Pachycondyla spp. (Hymenoptera, Formicidae, Ponerinae). Psyche Volume 2012, Article ID 973897, 9 pp. (doi:10.1155/2012/973897).
- Mariano, C.S.F., Santos, I.S., Silva, J.G., Costa, M.A., Pompolo, S.G. 2015. Citogenética e evolução do cariótipo em formigas poneromorfas. In: Delabie, J.H.C., Feitosa, R.M., Serrao, J.E., Mariano, C.S.F., Majer, J.D. (eds) As formigas poneromorfas do Brasil, 1st edn. Ilhéus, Brasil, pp 102–125 (doi:10.7476/9788574554419.0010).
- Marini-Filho, O.J. 1999. Distribution, composition, and dispersal of ant gardens and tending ants in three kinds of central Amazonian habitats. Tropical Zoology 12: 289-296.
- Orivel, J., Dejean, A. 1999. Selection of epiphyte seed by ant-garden ants. Ecoscience 6: 51-55.
- Orivel, J., Dejean, A. & Errard, C. 1998. Active role of two ponerine ants in the elaboration of ant gardens. Biotropica 30: 487-491.
- Orivel, J., Errard, C. & Dejean, A. 1997. Ant gardens: interspecific recognition in parabiotic ant species. Behavioral Ecology and Sociobiology 40: 87-93.
- Orivel, J., Leroy, C. 2011. The diversity and ecology of ant gardens (Hymenoptera: Formicidae; Spermatophyta: Angiospermae). Myrmecological News 14: 73-85.
- Orivel, J., Souchal A, Cerdan P, Dejean A. 2000. Prey capture behavior of the arboreal ponerine ant Pachycondyla goeldii. Sociobiology 35: 131-140.
- Rodrigues, M.S., Vilela, E.F., Azevedo, D.O., Hora, R.R. 2011. Multiple queens in founding colonies of the neotropical ant Pachycondyla striata Smith (Formicidae: Ponerinae). Neotropical Entomology 40, 293–299 (doi:10.1590/s1519-566x2011000300001).
- Santschi, F. 1920. Formicides africains et américains nouveaux. Annales de la Société Entomologique de France 88:361-390.
- Schmidt, C.A. & Shattuck, S.O. 2014. The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of ponerine ecology and behavior. Zootaxa 3817, 1–242 (doi:10.11646/zootaxa.3817.1.1).
- Troya, A., Marcineiro, F., Lattke, J.E. & Longino, J. 2022. Igaponera curiosa, a new ponerine genus (Hymenoptera: Formicidae) from the Amazon. European Journal of Taxonomy 823: 82–101 (doi:10.5852/ejt.2022.823.1817).
- Weber, N. A. 1944. The tree ants (Dendromyrmex) of South and Central America). Ecology 25:117-120
References based on Global Ant Biodiversity Informatics
- Astruc C., J. F. Julien, C. Errard, and A. Lenoir. 2004. Phylogeny of ants based on morphology and DNA sequence data. Molecular Phylogenetics and Evolution 31: 880-893.
- Dejean A., B. Corbara, J. Orivel, R. R. Snelling, J. H. C. Delabie, and M. Belin-Depoux. 2000. The importance of ant gardens in the pioneer vegetal formations of French Guiana (Hymenoptera: Formicidae). Sociobiology 35(3): 425-439.
- Fernández F. 2008. Subfamilia Ponerinae s.str. Pp. 123-218 in: Jiménez, E.; Fernández, F.; Arias, T.M.; Lozano-Zambrano F. H. (eds.) 2008. Sistemática, biogeografía y conservación de las hormigas cazadoras de Colombia. Bogotá: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, xiv + 609 pp.
- Franco W., N. Ladino, J. H. C. Delabie, A. Dejean, J. Orivel, M. Fichaux, S. Groc, M. Leponce, and R. M. Feitosa. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5): 509-543.
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Lachaud J. P., P. Cerdan, and G. Perez-Lachaud. 2012. Poneromorph ants associated with parasitoid wasps of the genus Kapala Cameron (Hymenoptera: Eucharitidae) in French Guiana. Psyche doi:10.1155/2012/393486.
- Leroy, C., B. Corbara, A. Dejean and R. Cereghino. 2009. Ants mediate foliar structure and nitrogen acquisition in a tank-bromeliad. New Phytologist 183(4): 1124-1133
- Mackay, W.P. and E.E. MacKay. 2010. The systematics and biology of the New World ants of the genus Pachycondyla (Hymenoptera: Formicidae). Edwin Mellen Press Lewiston, NY
- Orivel, J., A. Dejean and C. Errard. 1998. Active Role of Two Ponerine Ants in the Elaboration of Ant Gardens. Biotropica 30(3):487-491.

