Rhopalothrix diadema
| Rhopalothrix diadema | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Rhopalothrix |
| Species: | R. diadema |
| Binomial name | |
| Rhopalothrix diadema Brown & Kempf, 1960 | |
Other than a few brief specimen label remarks ("soil-leaf litter berlesate from lowland rain forest on the lower Busu River" col. Wilson, "secondary rainforest" col. Janda), nothing is known about the biology of Rhopalothrix diadema.
Identification
Keys including this Species
Distribution
Latitudinal Distribution Pattern
Latitudinal Range: -5.43751° to -8.766670227°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Indo-Australian Region: New Guinea.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
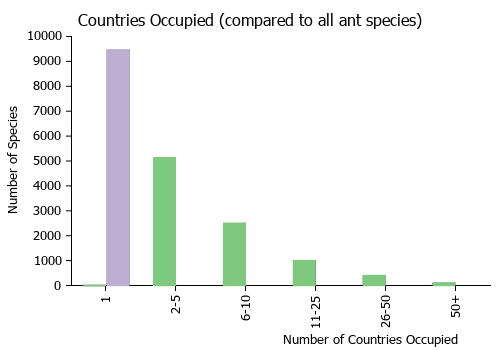
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
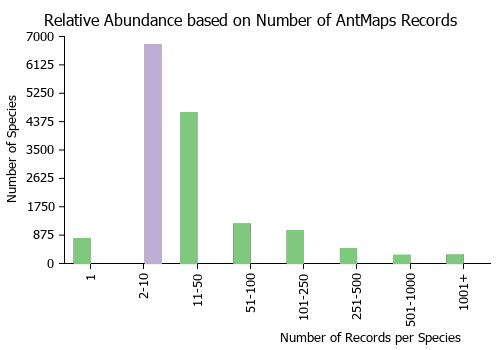
|
Biology
|
Castes
Known only from the worker caste.
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- diadema. Rhopalothrix diadema Brown & Kempf, 1960: 239, fig. 59 (w.) NEW GUINEA.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Holotype. TL 2.2, HL 0.52, HW 0.61 (CI 117), ML 0.22, WL 0.53 mm.
A very shallow sulcus crosses head just in front of the anterior arc of large round hairs. Eyes minute, each situated on underside of dorsal scrobe border and beneath one of the large hairs, so not visible from dorsal full-face view. Mandibles narrow, curved, with 4 or 5 spaced denticles; subapical tooth longer than basal width of mandible and slightly longer than (ventral) apical tooth; between subapical and apical teeth 2 blunt, indistinct intercalary denticles. Labrum flat, but with a large mediobasal tumulus (indicated by a dotted line in the figure) and a shallow anteromedian longitudinal sulcus leading to the tiny groove between the two narrow lobes, which together with their short, approximated sensilla, form an acute apex to the lateral shield. Antennal scapes convex above, but with a concave strip along the anterior edge.
Alitrunk compact, convex in outline, its summit at the posterior edge of mesonotum. A broad but shallow sulcus separating pro- and mesonotum, and a slight metanotal groove separating mesonotum from down-sloping propodeal dorsum. Seen from above, pronotum much broader than long, with rounded sides; mesonotum much smaller, suhcircular; distinct constriction at metanotal groove; sides of propodeum convex in front. Propodeum with median portion of surface continuous from dorsum to declivity and concave from side to side, guarded on each side by an obtuse angle (vestige of the propodeal tooth) trailing a low cariniform lamella below.
Petiole with a distinct but broadly conical peduncle and a rounded node, distinctly broader than long as seen from above. Postpetiole sub reniform, nearly twice as wide as petiolar node and about 2/3 as wide as widest part of gaster. Gaster oval, convex above and below, without a dorsal sulcus or impression.
Entire body densely and finely granulose-punctulate, opaque; promesonotum and petiole with fine superimposed rugulae. Ground pilosity consisting of round, white, subappressed squamiform hairs, abundant over head, scapes, labrum, promesonotum, legs, both nodes and gaster; largest on clypeus, pronotum, tibiae and gaster; smaller and inconspicuous on mandibles and petiolar node. Larger specialized hairs are shaped like the inverted bowls of broad, flat spoons lying close to and paralleling the integumantal surface; in perpendicular view, they look like large, round white scales: 18 on head, arranged as shown in Fig. 59; 2 pairs on mesonotum, 1 pair on posterior petiolar node, 1 pair on posterolateral corners of postpetiole; about 24 on first gastric tergite; 2 hairs at each tibial apex. Gastric apex with more or less erect clavate hairs both above and below. Color light ferruginous.
Type Material
Holotype worker Museum of Comparative Zoology taken in a soil-leaf litter berlesate from lowland rain forest on the lower Busu River, near Lae, New Guinea (E. O. Wilson leg., no. 1052). In the same Berlese sample were associated the ant species Eurhopalothrix biroi, E. brevicornis and Dacetinops cibdela Brown & Wilson.
Three paratype workers also from the same sample vary slightly in size and proportions: TL 2.1-2.4, HL 0.51-0.55, HW 0.59-0.65 (CI 116-118), ML 0.21-0.22, WL 0.52-0.56 mm, but are otherwise very similar. Paratypes in Museum of Comparative Zoology, National Museum of Natural History, WWK.
Longino and Boudinot (2013) - Holotype, worker: New Guinea, lower Busu River, near Lae, lowland rainforest (E. O. Wilson, no. 1052) Museum of Comparative Zoology (not examined).
- Paratype, 1 worker, lower Busu River, Huon Peninsula, Papua New Guinea, Wilson,E.O., ANIC32-017704, Australian National Insect Collection.
References
- Brown, W. L., Jr.; Kempf, W. W. 1960. A world revision of the ant tribe Basicerotini. Stud. Entomol. (n.s.) 3: 161-250 (page 239, fig. 59 worker described)
- Longino J. T. and Boudinot B. E. 2013. New species of Central American Rhopalothrix Mayr, 1870 (Hymenoptera, Formicidae). Zootaxa. 3616:301-324. doi:10.11646/zootaxa.3616.4.1
References based on Global Ant Biodiversity Informatics
- Brown W. L., Jr., and W. W. Kempf. 1960. A world revision of the ant tribe Basicerotini. Stud. Entomol. (n.s.) 3: 161-250.
- CSIRO Collection
- Janda M., G. D. Alpert, M. L. Borowiec, E. P. Economo, P. Klimes, E. Sarnat, and S. O. Shattuck. 2011. Cheklist of ants described and recorded from New Guinea and associated islands. Available on http://www.newguineants.org/. Accessed on 24th Feb. 2011.
- Longino J. T., and B. E. Boudinot. 2013. New species of Central American Rhopalothrix Mayr, 1870 (Hymenoptera, Formicidae). Zootaxa 3616: 301-324.
- Lucky A., E. Sarnat, and L. Alonso. 2011. Ants of the Muller Range, Papua New Guinea, Chapter 10. In Richards, S. J. and Gamui, B. G. (editors). 2013. Rapid Biological Assessments of the Nakanai Mountains and the upper Strickland Basin: surveying the biodiversity of Papua New Guineas sublime karst environments. RAP Bulletin of Biological Assessment 60. Conservation International. Arlington, VA.
- Snelling R. R. 1998. Insect Part 1: The social Hymenoptera. In Mack A. L. (Ed.) A Biological Assessment of the Lakekamu Basin, Papua New Guinea, RAP 9. 189 ppages

