Acromyrmex fowleri
| Acromyrmex fowleri | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Acromyrmex |
| Species: | A. fowleri |
| Binomial name | |
| Acromyrmex fowleri Rabeling, Messer, Lacau & Delabie, 2019 | |
A workerless social parasite of Acromyrmex rugosus. Evidence suggests this ant is an obligate parasite of its host and is tolerant of host queens. Acromyrmex fowleri was first discovered by Jacques H. C. Delabie in 1992. Delabie found winged queens and males of A. fowleri inside the nests of A. rugosus. These mixed colonies consisted of A. fowleri alates as well as a single dealate queen, many workers, and winged queens and males of A. rugosus.
| At a Glance | • Workerless Inquiline |
Identification
Rabeling et al. (2019) - Acromyrmex fowleri exhibits some morphological traits characteristic of the inquiline syndrome (sensu Kutter 1968; Wilson 1971; Hölldobler and Wilson 1990). Most notably, A. fowleri is significantly smaller than its host A. rugosus and the integumental sculpturing is completely reduced. Queens of A. fowleri are smooth and shiny. Superficially, A. fowleri resembles the socially parasitic fungus-growing ant species Pseudoatta argentina and Mycocepurus castrator. Acromyrmex fowleri retains four maxillary and two labial palp segments and 13 antennal segments that are the plesiotypic condition for fungus-growing ants.
Acromyrmex fowleri is remarkably similar to its host species, Acromyrmex rugosus. Notwithstanding, the gyne of A. fowleri can easily be distinguished from A. rugosus by the smaller size, the smooth and shiny integument, and the presence of appressed and transversally flattened setae. Relative to its smaller body size, A. fowleri is also characterized by longer appendages, and antennal scapes, as well as by a broader postpetiole.
In the field, A. fowleri can be distinguished from its host by the significantly smaller size, the shiny appearance, the distinctly orange–brown coloration, the slowness of its movements, and the occurrence of alate females and males in some host nests throughout the year, whereas alates of A. rugosus typically occur during the rainier and warmer summer months (December–March) in coastal Bahia.
The male of A. fowleri resembles the male of A. rugosus and is not gynaecomorphic. Large males of A. fowleri reach the same size as small and medium size of A. rugosus males, but never reach the body size of large A. rugosus males. Despite slightly overlapping size ranges, the males of A. folweri can be distinguished from the A. rugosus males by their shiny integument, the slenderer and less bulbous gaster, the relatively longer antennal scapes, the absence of the inferior pronotal spine, the more pronounced tubercles on the first gastral tergite, and the presence of few, dorsoventrally flattened, appressed setae. The parasites’ genitalia are smaller than the hosts’ genitalia (paramere length: A. fowleri = 1.04 mm, A. rugosus = 1.66 mm), the length of the ventral aedeagal lobe is smaller in A. rugosus (A. fowleri = 0.26 mm, A. rugosus = 0.21–0.22 mm). It is important to note that the aedeagus of A. rugosus bears a very large dorsal lobe that is absent from A. fowleri. The ventral border of the aedeagus bears 12 teeth in A. fowleri and 12–14 teeth in A. rugosus.
Distribution
Known from the coast of Ilhéus in the State of Bahia in northeastern Brazil.
Latitudinal Distribution Pattern
Latitudinal Range: -14.6197° to -14.781°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Brazil (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
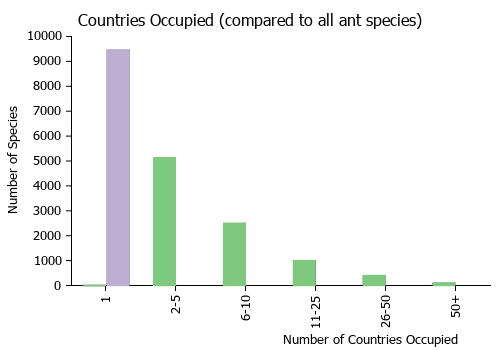
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
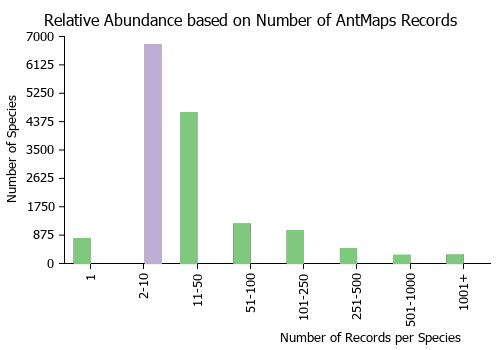
|
Biology
|
This ant is a workerless inquiline social parasite in the nest of Acromyrmex rugosus.
This biology section is based on Rabeling et al 2019, which brought together previously published and unpublished observations about this species and its host.
Colony data from excavated nests shows A. fowleri is polygnous and a host queen-tolerant inquiline social-parasite (Delabie et al. 2002). Alates of A. rugosus were also observed in parasitized colonies (Delabie et al. 1993), which shows the host of A. fowleri can be reproductively active in the presence of A. fowleri.
Acromyrmex fowleri exhibits some morphological traits characteristic of the inquiline syndrome (sensu Kutter 1968; Wilson 1971; Hölldobler and Wilson 1990). Most notably, A. fowleri is significantly smaller than its host A. rugosus and the integumental sculpturing is completely reduced. Queens of A. fowleri are smooth and shiny. Acromyrmex fowleri retains four maxillary and two labial palp segments and 13 antennal segments, characters that are the plesiotypic condition of fungus-growing ants.
Delabie et al. (1993) noted that nest-density was unusually high among parasitized A. rugosus populations, with nest entrances being approximately 10 meters apart (Delabie et al. 1993). Delabie and colleagues excavated a total of 65 nests and in five of them (7.7%) alate queens and males were detected (Delabie et al. 1993, 2002). For two parasitized colonies, a full colony census of parasite alates was taken, revealing 30 females and eight males in the first, as well as 38 females and ten males in the second host colony (Delabie et al. 1993). The numerical male–female sex ratio was biased towards females for both colonies (1:3.75 and 1:3.8).
A. fowleri seemed to have been a common species in the Ilhéus region at the time when behavioral observations were first conducted (1992–1996) with a host nest density estimated to be about 20 nests per hectare (Delabie, pers. observation). During the past 15 years, however, it seems A. fowleri have almost entirely disappeared from along the coast in Ilhéus . The last systematic attempt to find A. fowleri was made in 2004, when one of us (I. C. do Nascimento) collected alates from mating flights for the entire year from January to December along a 6-km beach transect, including one of the sites where A. fowleri was commonly encountered between 1993 and 1996. Only five A. fowleri alates were found during the year-long survey, while alates of the host were most regularly reported (I. C. do Nascimento et al. pers. observations). It is unknown whether the high parasite density caused the decline of the host species in this habitat, or whether the population density of A. rugosus declined for other reasons and is now too small to support a viable social parasite population. It is possible that A. fowleri is now locally extinct.
Mating flights
A. fowleri performs pre-dawn mating flights. Large numbers of alate A. fowleri queens and males were found during the morning hours (6–7 h) along the drift line on the beach at the Atlantic Ocean. This suggests mating occurs outside the host nest.
Mating flights of A. fowleri were observed year-round at a low rate; however, from October to March/April the number of dispersing alates increased significantly (Delabie et al. 2002). The majority of all alates (70%; 247 of 354 individuals) were collected during the month of November in 1994. Approximately, 13% (45 of 354 individuals) of alates were collected outside of the peak dispersal months, suggesting that low-frequency dispersal occurs throughout the year. The mating flights of parasite and host were not fully synchronized, but the time periods of their mating flights overlapped broadly, with A. rugosus dispersing from December to March (Delabie et al. 2002). The mating flights of A. fowleri and A. rugosus, therefore, coincide with the warmer and rainier austral summer, which is a frequently observed pattern for fungus-growing ants (Mehdiabadi and Schultz 2010). Most of the parasite behavior following the mating flight is unknown, but Fowler (Fowler, pers. observation; cited in Schultz et al. 1998) observed that newly mated A. fowleri queens were detected by host workers and repulsed from the nest when they tried to enter mature colonies of A. rugosus. This suggests A. rugosus is capable of detecting and evicting invading social parasites. It is currently unknown whether A. fowleri reproduces semelparously or iteroparously. Delabie’s observations that the fungus garden of parasitized A. rugosus colonies looked healthy and showed no evidence of colony decline provide circumstantial evidence for semelparous reproduction of the parasite, similar to Mycocepurus castrator (Rabeling and Bacci 2010).
Phylogenetic Analyses
A mitochondrial phylogeny of a subset of Acromyrmex species inferred A. fowleri as a distant relative to A. rugosus suggesting that A. fowleri evolved via allopatric speciation (Sumner et al. 2004). In contrast to this earlier study, our ongoing studies that utilize a complete taxon sampling of all Acromyrmex species and are based on multiple nuclear as well as genomic markers infer A. fowleri as a close relative of its host A. rugosus, suggesting that A. fowleri originated via the intraspecific, sympatric route of social parasite evolution (Rabeling et al. unpublished manuscript). Our molecular phylogenetic analyses are also consistent with the morphological analysis, showing that A. fowleri and A. rugosus share many key morphological characters.
Castes
An inquiline presumed to have no workers.
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- fowleri. Acromyrmex fowleri Rabeling, Messer, Lacau & Delabie, in Rabeling, Messer, Lacau, et al. 2019: 445, figs. 1a,c,e, 2a,c,e, 3, 4a,c,e (q.m.) BRAZIL (Bahia).
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Queen
holotype: TL 7.90, WL 2.47, HL 1.30, HW 1.45, IOD 1.59, ML 0.97, FLD 0.75, SL 1.59, EL 0.37, EW 0.33, PrW 1.38, FL 1.73, PL 0.52, PW 0.57, PPL 0.50, PPW 0.92, GL 2.15, CI 110, MI 73, SI 109.
paratype gynes (n = 25): TL 7.33–8.13, WL 2.34–2.69, HL 1.25–1.31, HW 1.38–1.47, IOD 1.53–1.61, ML 0.89–0.98, FLD 0.73–0.78, SL 1.51–1.71, EL 0.36–0.39, EW 0.33–0.37, PrW 1.23–1.44, FL 1.65–1.76, PL 0.48–0.58, PW 0.56–0.63, PPL 0.43–0.55, PPW 0.91–1.02, GL 1.90–2.22, CI 106–114, MI 69–76, SI 107–120.
A small species of Acromyrmex fungus-growing ants (WL 2.47, TL 7.90) that is immediately recognizable as a social parasite due to its smaller body size and shiny integument. Mandibles (MI 73) and appendages (FL 1.73, SI 109) long relative to head and body size, respectively. Integument smooth and shiny, in part translucent, characteristic of the inquiline syndrome. Body surface, antennal scapes, and legs (except for tarsi) sparsely covered with pale, transversally flattened, appressed setae; only tarsi covered with semi-erect setae. Color uniformly light orange brown; anepisternum, scutellum, and masticatory margin of mandibles slightly darker, reddish-brown. Head head shape subquadrate, slightly wider than long (CI 110); lateral margins parallel to each other; posterior margin with median concavity; posterior and lateral margins with numerous distinct tubercles. Mandibles long and slender in full-face view; external margins sinuate; masticatory margins with 12 teeth; apical and preapical teeth distinctly larger, followed by five small teeth that are interspersed by smaller denticles; mandible surface smooth and shiny, not striate. Palp formula 4:2, representing the plesiomorphic condition of fungus-growing ants. Posterior margin of clypeus trapezoidal, broadly inserted between frontal lobes; anterior margin of clypeus shiny and median portion concave. Unpaired median clypeal seta short (0.09 mm), semi-erect, transversally flattened, only barely projecting over the anterior clypeal margin. Frontal lobes broadly rounded, fully covering condylar bulbs in full-face view; lateral margin of frontal lobe serrated with two distinct tooth-like projections. Frontal carinae extending towards the level of the ocelli, not forming a fully shaped antennal scrobe. Preocular carina forming a straight line in lateral view and traversing the area of the antennal scrobe by onethird of the scrobe’s width behind the level of the eye. Eyes large (EL 0.37, EW 0.33) and strongly convex. The three ocelli are small and embedded in the integument. Antennae with 11 segments. Antennal scapes long (SL 1.59, SI 109) with abundant, appressed setae, surpassing the posterior margin of the head by more than one-third of its length. Mesosoma slender with caste-specific modifications related to wing bearing. Dorsolateral pronotal spine long, slender, and sharply pointed in dorsal view. Ventrolateral pronotal spine reduced, triangular, with blunt, rounded tip. Dorsum of mesosoma smooth and shiny. Posterior margin of scutellum concave, but not distinctly bidentate. Bulla and meatus of metapleural gland not notably modified from the condition in the host species. Propodeal spines straight, slender, sharply pointed, projecting away from the propodeum at a 90° angle in lateral view. Metasoma Anterior peduncle of petiole short, about one-fourth the length of the petiolar node. Dorsum of petiolar node with a pair of irregularly shaped ridges. Postpetiole wider (PPW 0.92) than long (PPL 0.50) in dorsal view, posterior margin slightly concave. Gaster large (GL 2.15). First gastric tergite notably smooth with few broadly rounded, reduced tubercles; on median portion of anterior half tubercles form a pair of shallow, longitudinal ridges. Except for the smaller size, foreand hindwings resemble the wings of the host species, A. rugosus.
Male
Paratype males (n = 25): TL 6.89–7.32, WL 2.25–2.47, HL 0.96–1.02, HW 1.00–1.07, IOD 1.25–1.33, ML 0.64–0.70, FLD 0.49–0.51, SL 1.31–1.45, EL 0.37–0.41, EW 0.38–0.43, PrW 1.23–1.44, FL 1.67–1.76, PL 0.37–0.44, PW 0.50–0.61, PPL 0.37–0.44, PPW 0.79–0.92, GL 2.15–2.47, CI 99–108, MI 64–71, SI 128-140.
A small male (WL 2.25–2.47, TL 6.89–7.32), distinctly smaller than the male of the host species, Acromyrmex rugosus. Integument smooth and shiny, thin, partly translucent. Body surface covered with few appressed, transversally flattened setae. Color uniformly pale yellowto reddish-brown. Head Approximately as wide as long (CI 99–108); behind the level of the eyes, sides rounded and tapering towards the posterior margin of head; head size small relative to mesosoma. Mandibles long, slender, with distinct apical and preapical teeth, followed by seven to eight smaller, irregularly spaced teeth of irregular size; mandible surface smooth, shiny. Palp formula 4:2. Clypeus shape as in gyne; unpaired clypeal seta projecting over the anterior clypeal margin by two-thirds its length. Frontal lobes narrow, leaving the anterior half of the condylar bulbs exposed in full-face view. Preocular carina distinct, traversing the area of the antennal scrobe almost completely, nearly touching frontal carina. Eyes very large (EL 0.37–0.41, EW 0.38–0.43), strongly convex. Ocelli large, raised above the surface of the head; unpaired median ocellus approximately one and a half times wider than paired lateral ocelli. Antennae with 13 segments. Antennal scape long (SL 1.31–1.45) with appressed setae, surpassing the posterior margin of the head by half its length. Mesosoma with sex-specific modifications related to wing bearing. Anteriodorsal portion of pronotum inflated. Dorsolateral pronotal spine short, broadly triangular, and blunt in dorsal view. Ventrolateral pronotal spine absent. Dorsum of mesosoma smooth. Scutellum shape as in gyne; sculpture granulate with fine rugae. Bulla and meatus of metapleural gland small, orifice of metapleural gland tiny and round, pointing posteriorly, not notably modified from the condition in A. rugosus. Propodeal spines short, narrowly triangular, sharply pointed. Metasoma: Petiole and postpetiole as in gyne. Gaster less bulbous than in host. First gastric tergite smooth, shiny, laterally with few reduced, rounded tubercles; sparsely covered with few appressed setae. Foreand hindwings resemble the wings of its host, except for smaller size and a missing detached vein at the posterior end of the hindwing. Genitalia In toto, excluding the basal ring, parameres longer (1.04 mm) than wide (0.83 mm); apical lobe of paramere evenly rounded with less than 10 long, erect setae. In lateral view, aedeagus small (0.26 mm), ventral border of penis valve bearing 12 recurved teeth of uniform length; the anterior two and posteriormost of which are small and weakly sclerotized, whereas teeth 3-11 are distinctly larger and heavily sclerotized, as notable by the darker brown coloration.
Type Material
- Holotype, queen, Bahia, Ilhéus, Praia do Norte, 18 km N Ilhéus, Brazil, 14°37′11″S 39°03′39″W / 14.6197°S 39.0607°W, 27 November 1994, Jacques Delabie, ASUSIBR00000001, Laboratório de Mirmecologia CEPEC / CPDC; collected from the drift line of the Atlantic Ocean on the beach.
It is presumed alates of A. fowleri participated in a predawn nuptial flight and drowned in the sea, which is why they may be found along the drift line of the beach.
Paratypes 279 alate gynes and 84 males, ASUSIBR00000002-00000364, from three collection sites along a 40 km stretch of “restinga” habitat (i.e., tropical and subtropical coastal forest habitats that form on sandy, acidic, nutrient-poor soils) along the coastline with the city of Ilhéus at its center (see the publication for many more collection details of these specimens)
Etymology
This inquiline social parasite is named in honor of our colleague, the late Harold Gordon Fowler, in recognition of his numerous important contributions to leaf-cutting ant biology, taxonomy, biogeography, and pest management in Brazil and Paraguay.
Determination Clarifications
Previous publications have referred to A. fowleri as “Pseudoatta new species”, “Pseudoatta from Bahia”, “Pseudoatta from Brazil”, and “Acromyrmex new species” (Delabie et al. 1993, Delabie et al. 2002; Schultz et al. 1998; Sumner et al. 2004; Schultz and Brady 2008; Rabeling and Bacci 2010; Soares et al. 2011; Rabeling et al. 2015).
References
- Barros, L.A.C., Aguiar, H.J.A.C., Teixeira, G.C., Souza, D.J., Delabie, J.H.C., Mariano, C.S.F. 2021. Cytogenetic studies on the social parasite Acromyrmex ameliae (Formicidae: Myrmicinae: Attini) and its hosts reveal chromosome fusion in Acromyrmex. Zoologischer Anzeiger 293, 273–281 (doi:10.1016/j.jcz.2021.06.012).
- Borowiec, M.L., Cover, S.P., Rabeling, C. 2021. The evolution of social parasitism in Formica ants revealed by a global phylogeny. Proceedings of the National Academy of Sciences 118, e2026029118 (doi:10.1073/pnas.2026029118).
- de la Mora, A., Sankovitz, M., Purcell, J. 2020. Ants (Hymenoptera: Formicidae) as host and intruder: recent advances and future directions in the study of exploitative strategies. Myrmecological News 30: 53-71 (doi:10.25849/MYRMECOL.NEWS_030:053).
- Rabeling, C. 2020. Social Parasitism. In: Starr, C. (ed.) Encyclopedia of Social Insects. Springer, Cham. (doi:10.1007/978-3-319-90306-4_175-1).
- Rabeling, C., Messer, S., Lacau, S., do Nascimento, I.C., Bacci, M., Delabie, J.H.C. 2019. Acromyrmex fowleri: a new inquiline social parasite species of leaf‑cutting ants from South America, with a discussion of social parasite biogeography in the Neotropical region. Insectes Sociaux 66: 435–451 (doi:10.1007/s00040-019-00705-z).
References based on Global Ant Biodiversity Informatics
- Rabeling C., S. Messer, S. Lacau, I. C. do Nascimento, M. Bacci Jr., and J. H. C. Delabie. 2019. Acromyrmex fowleri: a new inquiline social parasite species of leaf‑cutting ants from South America, with a discussion of social parasite biogeography in the Neotropical region. Insectes Sociaux https://doi.org/10.1007/s00040-019-00705-z



