Brachyponera chinensis
| Brachyponera chinensis | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Genus: | Brachyponera |
| Species: | B. chinensis |
| Binomial name | |
| Brachyponera chinensis (Emery, 1895) | |
| Synonyms | |
| |
| Common Name | |
|---|---|
| Asian Needle Ant | |
| Language: | English |
| Oo-hari-ari | |
| Language: | Japanese |
Buczkowski (2016) - The Asian needle ant, Brachyponera chinensis, is an invasive ant species introduced into the United States from Japan in the early 1930s (Smith 1934). Following the initial introduction, the species remained largely inconspicuous for several decades (McGown 2009). Recently, however, P. chinensis have become widespread in parts of the southeastern US and are now a common pest in urban and natural habitats (Guenard and Dunn 2010). In mature temperate forests, P. chinensis cause a strong decline in native ant abundance (Guenard and Dunn 2010) and disrupt ant-seed dispersal mutualisms by displacing native keystone ant species (Rodriguez-Cabal et al. 2012). In parts of North Carolina, USA, P. chinensis are displacing Linepithema humile by expanding their colonies early in the season (Spicer-Rice and Silverman 2013). Furthermore, recent predictive modeling demonstrates that climate change is going to significantly increase the global spread of P. chinensis by increasing the amount of habitat suitable to their invasion by 65 % worldwide (Bertelsmeier et al. 2013). The biology of P. chinensis is unique among invasive ants. First, while most invasive ants utilize carbohydrate-rich food sources consisting of floral nectar and hemipteran honeydew (Holway et al. 2002), P. chinensis is a predatory ant and a termite specialist (Bednar and Silverman 2011). There is no evidence that P. chinensis consumes nectar or hemipteran honeydew. Second, most invasive ants use mass recruitment via trail pheromones to collect food or toxic baits during management attempts. In contrast, no trail pheromones have been detected in P. chinensis. Instead, P. chinensis employs a unique yet relatively slow recruitment process called tandem carrying whereby foraging workers carry nestmates from the nest to the food source which is subsequently retrieved (Guenard and Silverman 2011). Finally, unlike colonies of many invasive ants which dominate urbanized and disturbed habitats, colonies of P. chinensis have the unique ability to invade habitats in undisturbed hardwood forests.
| At a Glance | • Highly invasive • Facultatively polygynous |
Photo Gallery
 A nest of Brachyponera chinensis. Photo by Minsoo Dong.
A nest of Brachyponera chinensis. Photo by Minsoo Dong.
Identification
Mackay and Mackay (2010): Brachyponera chinensis is an Old World species that was introduced into the New World. The combination of poorly developed mandibular teeth, the constriction at the metanotal suture and the form of the subpetiolar process, with a posteriorly directed lobe separate this species from all of the others present in the New World. Only the introduction of additional Old World species (at least in the New World) would make recognition of this species difficult.
Also see Brachyponera nakasujii for details about closely related species and the Caste section below for images that can help with determinations.
Distribution
Latitudinal Distribution Pattern
Latitudinal Range: 38.283333° to 9.2°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Australasian Region: New Zealand.
Indo-Australian Region: Brunei Darussalam, Philippines.
Nearctic Region: United States.
Oriental Region: Cambodia, Laos, Nepal, Taiwan, Thailand, Vietnam.
Palaearctic Region: China (type locality), Democratic Peoples Republic of Korea, Italy, Japan, Republic of Korea, Russian Federation.
Japan (Honshu, Shikoku, Kyushu, Nansei Is, Ogasawara Is).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Habitat
From Mackay and Mackay (2010):Smith (1979) reports they occur in dark damp habitats in urban environments (Smith, 1934) and disturbed rural environments (Brown, 1958).
Biology
As this ant is found in more areas, and is becoming known as a destructive invasive pest, it is becoming an increasingly focused on species of interest. A climate change analysis (Bertelsmeier et al. 2013) predicts global warming will greatly increase the potential areas where Brachyponera chinensis can invade. Its northern spread in eastern North America will perhaps not be as dramatic as predicted by climate alone. Its preferred prey, termites, are lacking in most of the putative areas of possible range expansion.
Mackay and Mackay (2010): Smith (1934), Koriba (1963) and Gotoh and Ito (2008) summarized the biology of this species. Small colonies were found in moist rotten wood or in the soil under stones, logs, debris, etc. (Smith, 1934). Sexuals were found in a nest in August in Norfolk, Virginia. Foragers were more active on cloudy days as compared with sunny days. They fed on dead insects, fish scraps and juices of decayed fruits lying on the ground. People at one unspecified locality claimed that they were occasionally stung. At the time of collection (spring, 1932) they were most common in the vicinity of the docks. They occurred over the entire town of Washington, North Carolina and to a lesser extent of Norfolk, Virginia. These ants are unusually common and successful in China and apparently feed on dead insects (Brown, 1958).
Eyer et al. (2018) found that a bottleneck at the population scale has not affected the diversity or the level of heterozygosity within colonies, as inbreeding is not a consequence of the founder event, but is due to mating between sibs that pre-existed in native populations. This suggests generations of sibmating in native populations might have pre-adapted B. chinensis as a successful invader, reducing inbreeding depression through purifying selection of deleterious alleles.
Heterick & Kitching (2022) collected this species in a pitfall trap within a lowland dipterocarp forest in Brunei.
Foraging/Diet
Bednar and Silverman (2011) studied the association of this ant with termites in North Carolina: Pachycondyla chinensis nests in close proximity to and consumes subterranean termites (Rhinotermitidae). P. chinensis do not occur in habitats lacking Rhinotermitidae. We suggest that subterranean termites are critical for P. chinensis success in new habitats. We demonstrate that P. chinensis is a general termite feeder, retrieving Reticulitermes virginicus five times more often than other potential prey near P. chinensis colonies. Odors produced by R. virginicus workers, as well as other potential prey, attract P. chinensis. Furthermore, P. chinensis occupy R. virginicus nests in the lab and field and display behaviors that facilitate capture of R. virginicus workers and soldiers. Termites are an abundant, high quality, renewable food supply, in many ways similar to the hemipteran honeydew exploited by most other invasive ant species. We conclude that the behavior of P. chinensis in the presence of termites increases their competitive abilities in natural areas where they have been introduced.
Buczkowski (2016) - Behavioral observations revealed that P. chinensis become visibly excited when a termite was present within approximately 2 cm away. The ants exhibited fast, erratic running and directed movement toward the termite. This suggests that termites emit volatile chemicals that are detected by P. chinensis that may help the ants locate their prey. Alternatively, P. chinensis may use visual cues in locating termites or a combination of visual and chemical cues.
Guénard and Silverman (2011) - Tandem carrying was previously described in Japanese by Takimoto (1988), however none of the reviews or studies on foraging recruitment published later considered this study (Beckers et al. 1989; Traniello 1989; Hölldobler and Wilson 1990; Baroni Urbani 1993; Passera and Aron 2006). In Brachyponera chinensis in North Carolina tandem carrying was first observed in June 2007. We later tested for the occurrence of this behavior near other B. chinensis colonies in four separate locations in Cary and Raleigh, NC by providing cockroaches, adult B. germanica L, to elicit recruitment. In all cases, tandem carrying was observed in response to food placement. This same behavior was also later observed in the native range of B. chinensis in Okayama, Japan. A successful tandem carry by B. chinensis comprises several steps. A scout returns to the nest following the discovery of food too large to be moved by a single individual. Upon return to the nest, the scout solicits a nestmate worker by drumming it with its antennae. The antennated worker assumes a pharate (pupal)-like posture with legs appressed to the thorax. The scout, now referred to as the carrier, then picks it up. The carrier holds the recruited worker within its mandibles between the worker’s first and second pairs of legs of the mesometasternum. The carried worker’s head is positioned upwards while being transported to the food, after which it is released directly adjacent to or nearly within a 2-cm radius of the food. Interestingly, the path taken by the tandem pair to the food is not linear but instead typically convoluted. Out of 28 observations, the carrier worker returned to the nest in 26 cases (93%) after the release of the carried worker but remained at the food in two cases. In most cases, carrier workers were observed turning around and inspecting the food prior to returning to the nest but without carrying any food themselves. We observed the dissection of large prey into smaller pieces, which were then transported to the nest by individual workers.
While tandem carrying appears ubiquitous within B. chinensis, expression of this behavior is context dependent. This is evidenced by the fact that tandem carrying occurred three to ten times more often with large nonmovable vs. small removable prey during peak foraging. We found no evidence for pheromone involvement in B. chinensis tandem-carrying recruitment. The mechanism by which the scout is able to return to the food and the mechanismby which the carried worker finds the nest are unresolved, although visual orientation cues may be employed (e.g., Jaffe et al. 1990; Collett and Collett 2002).
We consider tandem carrying as documented here in B. chinensis to be an original recruitment foraging strategy, perhaps the simplest yet described. In B. chinensis, the expression of this behavior is characterized by a graded recruitment and by high spatial and temporal flexibility. First, the number of tandem carrying events is resource dependent, with more recruitment to large prey that cannot be carried by a single worker than smaller movable prey, even at high density. Second, the recruitment observed by tandem carrying can be adjusted quickly in space and within a time period of 5 to 10 min to maximize the exploitation of larger prey. The low recruitment efficiency of this behavior seems to be balanced by a strong flexibility.
Colony Attributes
Gotoh and Ito (2008) studied a population in its native range: Mt. Yoshio-yama, Takamatsu-shi, Kagawa Prefecture, Shikoku Isl., western Japan. The abstract from their study: We investigated the seasonal cycle of changes in the colony structure of B. chinensis reproduced by alate queens in western Japan, and found the following novel biological characteristics of this species. B. chinensis showed a remarkable caste dimorphism in ovariole numbers: workers had no ovaries while queens had 18 to 36 ovarioles in their ovaries. The nesting system seemed to be polydomous: 266 of 400 nests collected were queenless. The number of queenless nests increased during the reproductive season. Among the 134 queenright nests, 38 had several mated-queens without significant differences in ovary activation and the remaining 96 nests were monogynous. During winter to early spring, most nests were polygynous. After alate production, most of the old queens seemed to die or be expelled and replaced by new queens. Virgin dealated queens were often found and they seemed to have laid eggs.
Nesting Habits
Allen et al. (2017) studied nesting relocation in a laboratory setting: "Subsets of B. chinensis worker ants were subjected to physical nest disturbance, and the recruitment methods and associated behaviors were recorded. Before recruitment to the new nest location began, B. chinensis ants organized into three distinctive groups: queen-tending, broodtending, and scouting. Once the new nest site was identified, scout ants began physically transporting nestmates into the new harborage. Transport rates increased with time in the first 30 minutes and did not change during the 30 to 55 minute interval when brood was transported. However, adult transport rate increased again after brood transport was completed and decreased after 90 minutes."
Flight Period
| X | X | X | |||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
Source: antkeeping.info.
- Check details at Worldwide Ant Nuptial Flights Data, AntNupTracker and AntKeeping.
- Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Ant Community Interactions
Guénard and Dunn (2010): Examined ant diversity and abundance in mature forests of North Carolina. Where it was present, P. chinensis was more abundant than all native species combined. The diversity and abundance of native ants in general and many individual species were negatively associated with the presence and abundance of P. chinensis. A small subset of species larger than P. chinensis (Camponotus and Formica) was either as abundant or even more abundant in invaded than in uninvaded sites. The large geographic range of this ant species combined with its apparent impact on native species make it likely to have cascading consequences on eastern forests in years to come.
Rodriguez-Cabal et al. (2012) in North Carolina, USA found B. chinensis was negatively correlated with the presence of Aphaenogaster rudis, decreased the seed removal rate of a myremchorous plant seed and was negatively correlated with a second forest plant that produces mymecochorous seeds: "The number of A. rudis workers was 96% lower in invaded than in intact plots, and the number of seeds removed was 70% lower in these plots. Finally, in invaded plots the abundance of Hexastylis arifolia, a locally abundant myrmecochorous plant, was 50% lower than in plots where P. chinensis was absent. A parsimonious interpretation of our results is that P. chinensis causes precipitous declines in the abundance of A. rudis within invaded communities, thereby disrupting the ant-plant seed dispersal mutualisms and reducing abundances of ant-dispersed plants.
Spicer Rice and Silverman (2013) found P. chinensis is displacing another invasive ant, Linepithema humile, in an urban habitat in Morriseville, North Carolina. The latter is at the northern limits of its range while the former, more adapted to cooler winters, is not at an edge of its invaded range. P. chinensis was found to be active up to two months before L. humile. Their findings suggests the lower thermal tolerance of the ponerine helps in establishing itself before L. humile begins their spring activity and this provides an important competitive advantage.
Warren et al. (2015) - In the deciduous forests of the north Georgia Piedmont the increasing abundance of B. chinensis is displacing the native Aphaenogaster rudis. B. chinensis was diminishing the abundance of A. rudis. Like the latter it was also preying upon, perhaps more effectively than A. rudis, on the termite Reticulitermes flavipes. In contrast to A. rudis B. chinensis was not serving as an effective seed disperser of myrmecochorous seeds in this forest habitat.
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
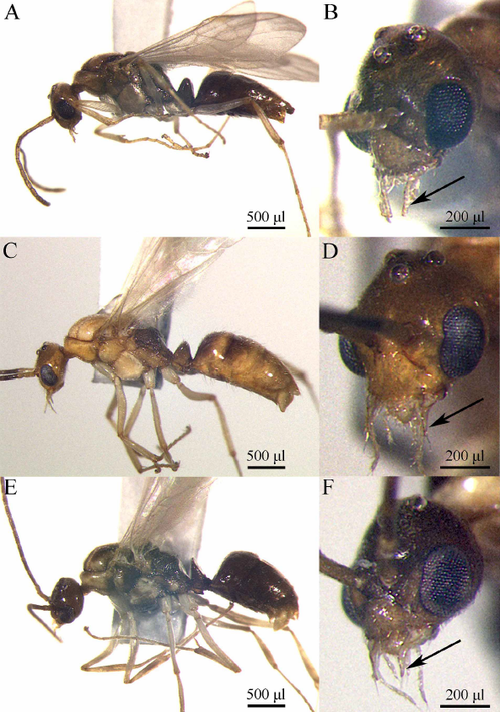
- This species is a host for the cestode Raillietina kashiwarensis (a parasitoid) (Quevillon, 2018) (encounter mode secondary; indirect transmission; transmission outside nest).
Castes
Worker
   
| |
| . | Owned by Museum of Comparative Zoology. |
Male
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- chinensis. Ponera nigrita subsp. chinensis Emery, 1895k: 460 (in text) (w.) CHINA (Shanghai).
- [Misspelled as sinensis by Imai & Kubota, 1972: 194.]
- Replacement name for Ponera solitaria Smith, F. 1874: 404. [Junior primary homonym of Ponera solitaria Smith, F. 1860b: 103.]
- [Note: chinensis junior synonym of solitaria Smith, F. 1874: 404 (synonymy by Brown, 1958h: 22); hence first available replacement name.]
- Wheeler, W.M. 1921c: 530 (q.); Ogata, 1987: 116 (m.); Wheeler, G.C. & Wheeler, J. 1986c: 88 (l.); Imai & Kubota, 1972: 194 (k.).
- Combination in Euponera (Brachyponera): Emery, 1909c: 367;
- combination in Pachycondyla: Brown, in Bolton, 1995b: 304;
- combination in Brachyponera: Brown, 1958h: 22; Schmidt, C.A. & Shattuck, 2014: 80.
- Subspecies of nigrita: Emery, 1909c: 367; Emery, 1911d: 84; Wheeler, W.M. 1921c: 530; Santschi, 1925f: 82; Wheeler, W.M. 1929f: 1; Wheeler, W.M. 1929g: 35; Wheeler, W.M. 1930h: 60; Baltazar, 1966: 244.
- Subspecies of luteipes: Wheeler, W.M. 1927h: 84; Wheeler, W.M. 1928c: 6; Chapman & Capco, 1951: 64.
- Junior synonym of luteipes: Wilson & Taylor, 1967: 103.
- Junior synonym of solitaria: Brown, 1958h: 22; Onoyama, 1980: 196; Terayama, 2009: 104.
- Status as species: Brown, 1958h: 22; Collingwood, 1976: 300; Azuma, 1977: 112; Onoyama, 1980: 196; Taylor, 1987a: 10; Morisita, et al. 1989: 19; Wang, M. 1992: 677; Xu, 1994b: 182; Bolton, 1995b: 304; Tang, J., Li, et al. 1995: 32; Kim, Kim & Kim, 1998: 147; Zhou, 2001b: 52; Zhang, W. & Zheng, 2002: 218; Imai, et al. 2003: 211; Lin & Wu, 2003: 67; Jaitrong & Nabhitabhata, 2005: 30; Radchenko, 2005b: 131; Don, 2007: 188; Terayama, 2009: 104; Mackay & Mackay, 2010: 247 (redescription); Yashiro, et al. 2010: 42; Zhou & Ran, 2010: 107; Ellison, et al. 2012: 91; Guénard & Dunn, 2012: 60; Jaitrong, Guénard, et al. 2016: 40.
Type Material
The following notes on F. Smith type specimens have been provided by Barry Bolton (details):
Ponera solitaria
Two worker syntypes in The Natural History Museum. Labelled “Japan. 74/16.” Acc. Reg.: “1874 no. 16. 2 Ponera solitaria. Hiogo (Japan). Presented by Fred. Smith. These insects were all collected by Mr Geo. Lewis, except those from Hokadadi which were collected by Mr Whiteley and Mr R. Fortune.” In the original description Smith gives the type-locality merely as “Hiogo.” Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Mackay and Mackay (2010): The worker is a small (total length 3.5 mm) brown specimen with yellowish brown mandibles, funiculi and legs. The mandibles have approximately 9 teeth, which alternate in size. The three apicalmost teeth are the largest with the first tooth approximately twice the length of the other two, which are approximately the same size. The transverse medial carina is poorly marked on the clypeus, the sides of the head are nearly parallel and the posterior margin is slightly concave. The head length is 0.88 mm; the head width is 0.75 mm. The eye is relatively large (maximum diameter 0.15 mm), located less than one diameter from the anterior margin of the head. The scape (0.85 mm) extends approximately two funicular segments past the posterior lateral corner of the head. The funicular segments are slightly swollen toward the apex, but do not form a club. The mesonotum is well defined on all sides and the mesosoma notably depressed at the metanotal suture. The propodeal spiracle is circular. The petiole is narrow when viewed in profile, with the anterior face being slightly concave near the apex and posterior face being slightly convex. Both faces narrow towards the apex and form a small horizontal dorsal surface. The subpetiolar process is a broad thick lobe, with a posteriorly directed sharp process. The metasternal process consists of two fang-like sharp elongate projections, similar to those found in members of the stigma species complex.
Erect hairs are sparse, but are present on the mandibles, clypeus, frontal lobes, a few hairs are present on the dorsum of the mesosoma, dorsum of the petiole and all surfaces of the gaster, a few hairs on the legs are erect. Very fine appressed sparse golden pubescence is found on most surfaces.
The mandibles are finely striated with scattered punctures the dorsum of the head is very finely and densely punctate and weakly shining, the dorsum of the mesosoma has similar sculpture. The sculpture on much of the side of the mesosoma, especially the side of the pronotum and mesopleuron and the lower part of the propodeum is smooth and glossy. The petiole has scattered punctures and is weakly shining; the gaster is sculptured and is slightly shinier.
Karyotype
- n = 11, 2n = 22, karyotype = 20M + 2A (Japan) (Imai & Kubota, 1972; Mariano et al., 2015) (as Brachyponera sinensis).
Etymology
The name means that this species is from China. (Mackay and Mackay 2010)
References
- Allen, H. R., P. A. Zungoli, E. P. Benson, and P. Gerard. 2017. Nest Emigration Behavior of the Asian Needle Ant, Brachyponera chinensis Emery (Hymenoptera: Formicidae). Sociobiology. 64:430-436 (doi:10.13102/sociobiology.v64i4.1586).
- Bednar, D. M. and J. Silverman. 2011. Use of termites, Reticulitermes virginicus, as a springboard in the invasive success of a predatory ant, Pachycondyla (=Brachyponera) chinensis. Insectes Sociaux. 58(4):459-476 (doi:10.1007/s00040-011-0163-0).
- Bertelsmeier, C., B. Guénard, and F. Courchamp. 2013. Climate change may boost the invasion of the Asian Needle Ant. PLoS ONE. 8(10): e75438:8 p. (doi:10.1371/journal.pone.0075438).
- Brown, W. L. 1958. A review of the ants of New Zealand. Acta Hymenopterologica 1:1-50.
- Brown, W. L., Jr. 1995a. [Untitled. Taxonomic changes in Pachycondyla attributed to Brown.] Pp. 302-311 in: Bolton, B. A new general catalogue of the ants of the world. Cambridge, Mass.: Harvard University Press, 504 pp. (page 304, Combination in Pachycondyla)
- Buczkowski, G. 2016. The Trojan horse approach for managing invasive ants: a study with Asian needle ants, Pachycondyla chinensis. Biological Invasions 18(2): 507-515 (doi:10.1007/s10530-015-1023-z).
- Davis, T. 2009. The ants of South Carolina (thesis, Clemson University).
- Dekoninck, W., Wauters, N., Delsinne, T. 2019. Capitulo 35. Hormigas invasoras en Colombia. Hormigas de Colombia.
- Del Toro, I., Robbons, R.R., Pelini, S.L. 2012. The little things that run the world revisited: a review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecological News 17: 133-146.
- Emery, C. 1895m. Viaggio di Leonardo Fea in Birmania e regioni vicine. LXIII. Formiche di Birmania del Tenasserim e dei Monti Carin raccolte da L. Fea. Parte II. Ann. Mus. Civ. Stor. Nat. 34[=(2(14): 450-483 (page 460, worker described)
- Emery, C. 1909d. Beiträge zur Monographie der Formiciden des paläarktischen Faunengebietes. (Hym.) Teil VIII. Dtsch. Entomol. Z. 1909: 355-376 (page 367, Combination in Euponera (Brachyponera))
- Esteves, F.A., Fisher, B.L. 2021. Corrieopone nouragues gen. nov., sp. nov., a new Ponerinae from French Guiana (Hymenoptera, Formicidae). ZooKeys 1074, 83–173 (doi:10.3897/zookeys.1074.75551).
- Eyer P-A, Matsuura K, Vargo EL, Kobayashi K, Yashiro Y, Suehiro W, Himuro C, Yokoi T, Guénard B, Dunn RR, Tsuji K (2018) Inbreeding tolerance as a pre-adapted trait for invasion success in the invasive needle ant Brachyponera chinensis. Molecular Ecology, 27, 4711-4724.
- Gochnour, B.M., Suiter, D.R., Booher, D. 2019. Ant (Hymenoptera: Formicidae) fauna of the Marine Port of Savannah, Garden City, Georgia (USA). Journal of Entomological Science 54, 417-429 (doi:10.18474/jes18-132).
- Gotoh, A. and F. Ito. 2008. Seasonal cycle of colony structure in the ponerine ant Pachycondyla chinensis in western Japan (Hymenoptera, Formicidae). Insectes Sociaux. 55(1):98-104 (doi:10.1007/s00040-007-0977-y).
- Guénard, B., Dunn, R.R. 2010. A new (old), invasive ant in the hardwood forests of Eastern North America and its potentially widespread impacts. PLoS ONE. 5(7): e11614:10 p. (doi:10.1371/journal.pone.0011614).
- Hashimoto, Y. 1990. Unique features of sensilla on the antennae of Formicidae (Hymenoptera). Applied Entomology and Zoology 25: 491-501.
- Hisasue, Y. 2018. Ant fauna of Matsuyama Castle. ARI 39: 18-36.
- Hisasue, Y. 2020. A checklist of the ants of Mt. Hiko-san (Kyushu, Japan). Korasana 93: 31-38.
- Imai, H. T.; Kubota, M. 1972. Karyological studies of Japanese ants (Hymenoptera, Formicidae) III. Karyotypes of nine species in Ponerinae, Formicinae and Myrmicinae. Chromosoma (Berl.) 37: 193-200 (page 194, karyotype described)
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87 (doi:10.3897@jhr.70.35207).
- Iwata, K., Eguchi, K., Yamane, S. 2005. A case study on urban ant fauna of southern Kyusyu, Japan, with notes on a new monitoring protocol (Insecta, Hymenoptera, Formicidae). Journal of Asia-Pacific Entomology 8, 263-272.
- Khachonpisitsak, S., Yamane, S., Sriwichai, P., Jaitrong, W. 2020. An updated checklist of the ants of Thailand (Hymenoptera, Formicidae). ZooKeys 998, 1–182 (doi:10.3897/zookeys.998.54902).
- Koriba, O. 1963. Colony founding of a female of Brachyponera chinensis in the observation cage. Kontyû 31:285-289 [in Japanese].
- Lee, C.-C., Weng, Y.-M., Lai, L.-C., Suarez, A.V., Wu, W.-J., Lin, C.-C., Yang, C.-C.S. 2020. Analysis of recent interception records reveals frequent transport of arboreal ants and potential predictors for ant invasion in Taiwan. Insects 11, 356 (doi:10.3390/INSECTS11060356).
- Liu, C., Fischer, G., Hita Garcia, F., Yamane, S., Liu, Q., Peng, Y.Q., Economo, E.P., Guénard, B., Pierce, N.E. 2020. Ants of the Hengduan Mountains: a new altitudinal survey and updated checklist for Yunnan Province highlight an understudied insect biodiversity hotspot. ZooKeys 978, 1–171 (doi:10.3897/zookeys.978.55767).
- Mackay, W.P., Mackay, E.E. 2010. The systematics and biology of the New World ants of the genus Pachycondyla (Hymenoptera: Formicidae). Edwin Mellon Press, Lewiston.
- Mariano, C.S.F., Santos, I.S., Silva, J.G., Costa, M.A., Pompolo, S.G. 2015. Citogenética e evolução do cariótipo em formigas poneromorfas. In: Delabie, J.H.C., Feitosa, R.M., Serrao, J.E., Mariano, C.S.F., Majer, J.D. (eds) As formigas poneromorfas do Brasil, 1st edn. Ilhéus, Brasil, pp 102–125 (doi:10.7476/9788574554419.0010).
- Nakajima, T. 2018. Evaluation of ant diversity in urban areas and research on IPM. Japanese Journal of Environmental Zoology 29, 149–158.
- Ogata, K. 1987a. A generic synopsis of the poneroid complex of the family Formicidae in Japan (Hymenoptera). Part 1. Subfamilies Ponerinae and Cerapachyinae. Esakia 25: 97-132 (page 116, male described)
- Park, S.-H., Hosoishi, S., Ogata, K. 2014. Long-term impacts of Argentine ant invasion of urban parks in Hiroshima, Japan. Journal of Ecology and Environment 37, 123–129 (doi:10.5141/ecoenv.2014.015).
- Park, S.-H., Hosoishi, S., Ogata, K., Kasuya, E. 2014. Changes of species diversity of ants over time: A case study in two urban parks. Journal of the Faculty of Agriculture, Kyushu University 59(1), 71–76.
- Park, S.-H., Hosoishi, S., Ogata, K., Kuboki, Y. 2014. Clustering of ant communities and indicator species analysis using self-organizing maps. Comptes Rendus Biologies 337, 545–552 (doi:10.1016/j.crvi.2014.07.003).
- Richter, A., Hita Garcia, F., Keller, R.A., Billen, J., Economo, E.P., Beutel, R.G. 2020. Comparative analysis of worker head anatomy of Formica and Brachyponera (Hymenoptera: Formicidae). Senckenberg Gesellschaft für Naturforschung 78(1), 133–170 (doi:10.26049/ASP78-1-2020-06).
- Rodriguez-Cabal, M. A., K. L. Stuble, B. Guénard, R. R. Dunn, and N. J. Sanders. 2012. Disruption of ant-seed dispersal mutualisms by the invasive Asian needle ant (Pachycondyla chinensis). Biological Invasions. 14(3):557-565 (doi:10.1007/s10530-011-0097-5).
- Ryu, J., Kim, Y.-K., Suh, S.J., Choi, K.S. 2021. The Insect database in Dokdo, Korea: An updated version in 2020. Biodiversity Data Journal 9, e62011 (doi:10.3897/bdj.9.e62011).
- Saito-Morooka, F., Fukuhara, K., Suda, K. 2015. The ant fauna of Rissho University at Kumagaya, Saitama Prefecture (Insecta, Hymenoptera, Formicidae). Bulletin of geo-environmental science (17), 35-39.
- Schifani, E. (2022). The new checklist of the Italian fauna: Formicidae. Biogeographia – The Journal of Integrative Biogeography 37, ucl006 (doi:10.21426/b637155803).
- Schmidt, C.A. & Shattuck, S.O. 2014. The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of ponerine ecology and behavior. Zootaxa 3817, 1–242 (doi:10.11646/zootaxa.3817.1.1).
- Siddiqui, J.A., Bamisile, B.S., Khan, M.M., Islam, W., Hafeez, M., Bodlah, I., Xu, Y. 2021. Impact of invasive ant species on native fauna across similar habitats under global environmental changes. Environmental Science and Pollution Research 28(39), 54362–54382 (doi:10.1007/s11356-021-15961-5).
- Smith, D. 1979. Formicoidea. In: Catalogue of Hymenoptera in America North of Mexico, K. Krombein, P. Hurd, D. Smith and B. Durks (eds). Volume 2: 1323-1467. Smithsonian Institute, Washington D. C.
- Smith, F. 1860b. Catalogue of hymenopterous insects collected by Mr. A. R. Wallace in the islands of Bachian, Kaisaa, Amboyna, Gilolo, and at Dory in New Guinea. J. Proc. Linn. Soc. Lond. Zool. 5(17b)(suppl. to vol. 4 4: 93-143 (page 103, junior primary homonym of solitaria)
- Smith, F. 1874b. Descriptions of new species of Tenthredinidae, Ichneumonidae, Chrysididae, Formicidae, &c. of Japan. Trans. Entomol. Soc. Lond. 1874: 373-409 (page 404, junior synonym of solitaria)
- Smith, M. R. 1934. Ponerine ants of the genus Euponera in the United States. Annals of the Entomological Society of America 27:557-564.
- Spicer Rice, E. and J. Silverman. 2013. Propagule pressure and climate contribute to the displacement of Linepithema humile by Pachycondyla chinensis. PLoS ONE. 8(2): e56281:11 p. (doi:10.1371/journal.pone.0056281).
- Subedi, I.P., Budha, P.B., Bharti, H., Alonso, L. 2020. An updated checklist of Nepalese ants (Hymenoptera, Formicidae). ZooKeys 1006, 99–136 (doi:10.3897/zookeys.1006.58808).
- Subedi, I.P., Budha, P.B., Bharti, H., Alonso, L., Yamane, S. 2023. Ponerine ants of Nepal (Hymenoptera: Formicidae, Ponerinae): a generic synopsis, new faunal records, and rediscovery of a rare ant, Emeryopone franzi (Baroni Urbani 1975). (doi:10.20362/am.016003).
- Suehiro, W., Hyodo, F. et al. 2017. Radiocarbon analysis reveals expanded diet breadth associates with the invasion of a predatory ant. Scientific Reports 7: 15016 (doi:10.1038/s41598-017-15105-1).
- Troya, A., Marcineiro, F., Lattke, J.E. & Longino, J. 2022. Igaponera curiosa, a new ponerine genus (Hymenoptera: Formicidae) from the Amazon. European Journal of Taxonomy 823: 82–101 (doi:10.5852/ejt.2022.823.1817).
- Warren, R. J., A. McMillan, J. R. King, L. Chick, and M. A. Bradford. 2015. Forest invader replaces predation but not dispersal services by a keystone species. Biological Invasions. 17:3153-3162 (doi:10.1007/s10530-015-0942-z).
- Warren, R.J., Chick, L. 2013. Upward ant distribution shift corresponds with minimum, not maximum, temperature tolerance. Global Change Biology 19, 2082–2088 (doi:10.1111/GCB.12169).
- Waters, J.S., Keough, N.W., Burt, J., Eckel, J.D., Hutchinson, T., Ewanchuk, J., Rock, M., Markert, J.A., Axen, H.J., Gregg, D. 2022. Survey of ants (Hymenoptera, Formicidae) in the city of Providence (Rhode Island, United States) and a new northern-most record for Brachyponera chinensis (Emery, 1895). Check List 18(6), 1347–1368 (doi:10.15560/18.6.1347).
- Wheeler, G. C.; Wheeler, J. 1986c. Supplementary studies of ant larvae: Ponerinae. Trans. Am. Entomol. Soc. 112: 85-94 (page 88, larva described)
- Wheeler, W. M. 1921c. Chinese ants. Bulletin of the Museum of Comparative Zoology 64: 529-547 (page 530, queen described)
- Yashiro, T.; Matsuura, K.; Guénard, B.; Terayama, M.; Dunn, R. R. 2010. On the evolution of the species complex Pachycondyla chinensis (Hymenoptera: Formicidae: Ponerinae), including the origin of its invasive form and description of a new species. Zootaxa 2685:39-50. [2010-11-24]
- Yu, Y. 2016. Risk of alien species introduction to Ogasawara Islands : Case study of ants at Tokyo Port. World Heritage Studies 1, 86-89.
- Zhao, Y., Sanders, N.J., Liu, J., Jin, T., Zhou, H., Lu, R., Ding, P., Si, X. 2021. β diversity among ant communities on fragmented habitat islands: the roles of species trait, phylogeny and abundance. Ecography 44, 1568–1578 (doi:10.1111/ecog.05723).
References based on Global Ant Biodiversity Informatics
- Abe A. 2006. Effect of thinning the artificial forests on the species composition of ants living in forest floor. Yahagigawa-kenkyu. 10:105108
- Abe T. 1971. On the food sharing among four species of ants in a sandy grassland. I. Food and foraging behaviour. Japanese Journal of Ecology 20(6): 219-230.
- Abe T. 1977. A preliminary study on the ant fauna of the Tokara Islands and Amami-Oshima. Ecol. Stud. Nat. Cons. Ryukyu Isl. 3: 93-102.
- Azuma M. 1938. A list of ants found in Osaka Prefecture, Japan. Entomological World. Tokyo 6:238-243.
- Azuma M. 1953. On the myrmecological fauna of Mt. Rokko, Hyogo Prefecture. Warera 2:1-7.
- Azuma, S. and M. Kinjo. 1987. Family Formicidae, In Checklist of the insects of Okinawa. The Biological Society of Okinawa, Nishihara. Pages 310-312.
- Chapman, J. W., and Capco, S. R. 1951. Check list of the ants (Hymenoptera: Formicidae) of Asia. Monogr. Inst. Sci. Technol. Manila 1: 1-327
- Choe L. J., K. J. Cho, S. K. Choi, S. H. Lee, M. K. Kim, H. S. Bang, J. Eo, and M. H. Kim. 2016. Effects of landscape and management on ground-dwelling insect assemblages of farmland in Jeju Island, Korea. Entomological Research 46: 36–44.
- Choi B.-M. 1987. Taxonomic study on ants (Formicidae) in Korea (1). On the genus Monomorium. Journal of the Institute of Science Education (Cheongju National Teachers' College) 11:17-30.
- Choi B.M. 1985. Study on distribution of ants (Formicidae) from Korea (1). Formic fauna in Mt. Songni. Cheongju Sabom Taehak Nonmunjip (Journal of Cheongju National Teachers' College) 22:401-437.
- Choi B.M. 1986. Studies on the distribution of ants (Formicidae) in Korea. Journal of Chongju National Teacher College 23: 317-386.
- Choi B.M. 1988. Studies on the distribution of ants (Formicidae) in Korea (5) Ant fauna in Is. Kanghwado. Chongju Sabom Taehak Nonmunjip (Journal of Chongju National Teacher' College) 25: 217-231.
- Choi B.M. 1996. Studies on the distribution of ants (Formicidae) in Korea (15) -Ant fauna islands Ullungdo and Dokdo. Journal of Chongju National University of Education 33: 201-219.
- Choi B.M. 1997. Distribution of Ants (Formicidae) in Korea (18). Ants Fauna in island Paekryongdo and Taechongdo. Journal of Chongju National University of Education 34: 119-138.
- Choi B.M. and C.H. Kim, 1987, Studies on the distribution of ants (Formicidae) in Korea (4). Ant fauna in Is. Hongdo and Is. Taehukusando. Journal of Chongju National Teacher College 24: 357-370.
- Choi B.M., K. Ogata, and M. Terayama. 1993. Comparative studies of ant faunas of Korea and Japan. 1. Faunal comparison among islands of Southern Korean and northern Kyushu, Japan. Bull. Biogeogr. Soc. Japan 48(1): 37-49.
- Choi B.M., Kim, C.H., Bang, J.R. 1993. Studies on the distribution of ants (Formicidae) in Korea (13). A checklist of ants from each province (Do), with taxonomic notes. Cheongju Sabom Taehakkyo Nonmunjip (Journal of Cheongju National University of Education) 30: 331-380.
- Choi B.M., and J. R. Bang. Studies on the distribution of ants (Formicidae) in Korea (12): the analysis of ant communities in 23 islands. Journal of Cheongju National University of Education 30:317-330.
- Collingwood C. A. 1976. Ants (Hymenoptera: Formicidae) from North Korea. Annales Historico-Naturales Musei Nationalis Hungarici 68:
- Emery C. 1911. Hymenoptera. Fam. Formicidae. Subfam. Ponerinae. Genera Insectorum 118: 1-125.
- Eto S., and K. Ogata. 1983. Ants of Hirado Island, Kyushu. Bulletin of the Nagasaki Prefecture Biological Group 25: 7-11.
- Fukumoto S. and Sk. Yamane. 2015. Records of ants from Uke–jima, Amami Islands, Japan (Hymenoptera, Formicidae). Nature of Kagoshima 41: 195–197.
- Gotoh, A. and F. Ito. 2008. Seasonal cycle of colony structure in the Ponerine ant Pachycondyla chinensis in western Japan (Hymenoptera, Formicidae). Insectes Sociaux 55(1): 98-104.
- Guénard B., J. K. Wetterer, and J. A. MacGown. 2018. Global and temporal spread of a taxonomically challenging invasive ant: the Asian needle ant, Brachyponera chinensis (Hymenoptera: Formicidae). Florida Entomologist 104: 649-656.
- Guénard B., and R. R. Dunn. 2012. A checklist of the ants of China. Zootaxa 3558: 1-77.
- Ha S.J, S.J. Park, and B.J. Kim. 2002. Comparative ant faunas between Seonyudo and seven other islands of West Sea in Korea. Korean Journal of Entomology 32(2): 75-79.
- Harada Y. 1997. Ants from the Koshiki islands, Kagoshima-ken, southern Japan. Ari 21: 1-4.
- Harada Y. 2000. Ant fauna of the forest floor of the Koshikijima Islands, Kagoshima-ken, southern Japan. Ari 24: 4-11.
- Harada Y., D. Fukukura, R. Kurisu, and S. Yamane. 2013. Ants of Ports, monitoring of alien ant species. Bull. Biogeogr. Soc. Japan 68: 29-40.
- Harada Y., H. Yadori, M. Yoneda, R. Takinami, K. Nagahama, Y. Matsumoto, A. Oyama, S. Maeda, and S. Yamane. 2009. Ant fauna of Tanegashima (Hymenoptera, Formicidae). Nankiseibutu, the Nanki Biological Society 51(1): 15-21.
- Harada Y., K. Asai, M. Araba, T. Higasayama, and N. Saito. 2019. Ant fauna at ports on the Goto Islands – monitoring of alien ant species –. Nature of Kagoshima 46: 27–32.
- Harada Y., K. Tashiro, K. Ebihara, H. Yadori, M. Yoneda, R. Takinami, K. Nagahama, and K. Hayashi. 2008. Ant fauna of the lavas of Sakurajima Volcano, Southern Japan. Bull. Biogeogr. Soc. Japan 63: 205-215.
- Harada Y., M. Enomoto, K. Nishimuta, and H. Mizumata. 2015. Ants of the Amami Islands, central Ryukyus, Japan. Nature of Kagoshima 41: 199–208.
- Harada Y., M. Enomoto, N. Nishimata, and K. Nishimuta. 2014. Ants of the Tokara Islands, northern Ryukyus, Japan. Nature of Kagoshima 40: 111121.
- Harada Y., S. Haruguchi, T. Iwasaki, K. Onishi, Y. Tashiro, and Sk Yamane. 2010. Ants from Japanese cherry trees, Prunus x yedoensis, in public parks in Kagoshima, southwestern Japan. Bull. Biogeogr. Soc. Japan 65: 169-179.
- Harada Y., Y. Matsumoto, S. Maeda, A. Oyama, and S. Yamane. 2009. Comparison of ant fauna among different habitats of Yaku-shima Island, southern Japan. Bull. Biogeogr. Soc. Japan 64: 125-134.
- Herwina H., and K. Nakamura. 2007. Ant species diversity study using pitfall traps in a small yard in Bogor Botanic garden, West Java, Indonesia. Treubia 35: 99-116.
- Hosoichi S., M. M. Rahman, T. Murakami, S. H. Park, Y. Kuboki, and K. Ogata. 2019. Winter activity of ants in an urban area of western Japan. Sociobiology 66(3): 414-419.
- Hosoichi S., M. Yoshimura, Y. Kuboki, and K. Ogata. 2007. Ants from Yakushima Island, Kagoshima Prefecture. Ari 30: 47-54.
- Hosoichi S., W. Tasen, S. H. Park. A. Le Ngoc, Y. Kuboki, and K. Ogata. 2015. Annual fire resilience of ground-dwelling ant communities in Hiraodai Karst Plateau grassland in Japan. Entomological Science 18: 254–261.
- Hosoishi S. 2006. Ant fauna of Noko Island. pp99-107. In: The floristic and faunistic surveys of the Noko Island.
- Hosoishi S., M. Yoshimura, Y. Kuboki, and K. Ogata. 2007. Ants from Yakushima Island , Kagoshima Prefecture. Ari 30: 47-54.
- Huong N. T. T., P. V. Sang, and B. T. Viet. 2015. A preliminary study on diversity of ants (Hymenoptera: Formicidae) at Hon Ba Nature Reserve. Environmental Scientific Conference 7: 614-620.
- Ichikawa A. 1999. Records of ants observed from several localities of Osaka Prefecture, Japan, -1. Ari 23: 1-3.
- Ikeshita Y., A. Gotoh, K. Yamamoto, N. Taniguchi, and F. Ito. 2007. Ants collected in Mt. Linoyama, Marugame, Kagawa Prefecture (Hymenoptera, Formicidae). Kagawa Seibutsu 34: 59-62.
- Ito F., and M. Minato. 1996. Ants collected in Kouchi-shi and Tosayokonami, Kouchi Prefecture. Ari 20:5-8.
- Ito, F.; Yamane, S.; Eguchi, K.; Noerdjito, W. A.; Kahono, S.; Tsuji, K.; Ohkawara, K.; Yamauchi, K.; Nishida, T.; Nakamura, K. 2001. Ant species diversity in the Bogor Botanic Garden, West Java, Indonesia, with descriptions of two new species of the genus Leptanilla (Hymenoptera, Formicidae). Tropics 10:379-404.
- Ito. F., Kondoh. M., Kubota. S., Masuko. K., Morishita. M., Murata. K., Ogata. K., Sato. T., Takamine. H., Yamaoka. H. and Kondoh. M. 1986. A list of ants collected at Akiyoshi-dai (Yamaguchi-ken) by the members of the Myrmecologists Society (Japan) in 1985. ARI Reports of the Myrmecologists Society (Japan) 14: 5-6
- Jaitrong W., and T. Ting-Nga. 2005. Ant fauna of Peninsular Botanical Garden (Khao Chong), Trang Province, Southern Thailand (Hymenoptera: Formicidae). The Thailand Natural History Museum Journal 1(2): 137-147.
- Jaitrong W.; Nabhitabhata, J. 2005. A list of known ant species of Thailand. The Thailand Natural History Museum Journal 1(1): 9-54.
- Kanai K., and S. Yamane. 2017. Myrmecological survey in an area destroyed by a pyroclastic flow of the Kuchinoerabu-jima volcano, South Japan. Nature of Kagoshima 43: 281–285.
- Kawahara Y., S. Hosoyamada, and S. Yamane. 1999. Ant fauna of the Terayama Station for Education and Research on Nature, Kagoshima University. Bulletin of the Faculty of Education, Kagoshima University. Natural Science 50: 147-156.
- Kim B., Ryu D., Park S., and J. Kim. 1994. Systematic study on ants from coasts of Korean Peninsula (Hym: Formicidae). Korean journal of entomology 24: 293-309.
- Kim B.J. 1996. Synonymic list and distribution of Formicidae (Hymenoptera) in Korea. Entomological Research Bulletin Supplement 169-196.
- Kim B.J., K.G. Kim, D.P. Ryu, J.H. Kim. 1995. Ants of Chindo island in Korea (Hymenoptera; Formicidae). The Korean Journal of Systematic Zoology 11(1): 101-113.
- Kim B.J., K.G. Kim, J.H. Kim, S.J. Park. Ants from Mt. Mirok. Korean J. Soil Zoology 2(2): 115-128.
- Kim B.J.; Kim, J.H.; Kim K 1998. Systematic study of Ponerinae (Hymenoptera: Formicidae) from Korea. Korean Journal of Entomology 28:145-154.
- Kim C.H., B.M. Choi, and J.R. Bang. 1992. Studies on the distribution of ants (Formicidae) in Korea (8)-Ant fauna in 10 islands, Chollanam-do. Korean J. Appl. Entomol. 31(4): 345-359.
- Kim K.I., C.H. Kim, and B. Choi. 1989. The ant fauna of the southern shore in Gyeongsangnamdo, Korea. Journal of Gyeongsang Nat. Univ. 28(2): 213-226.
- Kim et al. 1993. Systematic study of ants from Chejudo Province. Koran Journal of Entomology 23(3): 117-141.
- Kim, Byung-Jin, Ky-Gyong Kim, Dong-Pyo Ryu and Joong-Hyon Kim. 1995. The Korean Journal of Systematic Zoology. 11(1):101-113.
- Kondoh M., and Y. Kitazawa. 1984. Ant communities of the campus of UOEH and in an adjacent natural forest. Journal of UOEH 6(3): 221-234.
- Kubota S., and M. Terayama. 1982. Ant fauna of Kanagawa Prefecture, Japan (IV) Ants of Kakio. Kanagawa-chuho (Journal of the Kanagawa Entomologists Association) 21-28.
- Kubota S.; Terayama, M. 1989. Ant fauna of Tokyo. (1) A list of ants collected at the parks. Ari 16:14-16.
- Kubota. S., and M. Terayama. 1988. Ant fauna of Tokyo. (1) A list of ants collected at the parks. ARI Reports of the Myrmecologists Society (Japan) 16: 14-16
- Kwon T. S. 2012. Korean ant atlas. Korea Forest Research Institute 162 pages.
- Kwon T. S., S. S. Kim, and J. H. Chun. 2014. Pattern of ant diversity in Korea: An empirical test of Rapoport's altitudinal rule. Journal of Asia-Pacific Entomology 17: 161167.
- Kwon T.S., C. M. Lee, J. H. Chun, J. H. Sung, and S. K. Kim. 2011. Ants in Hongneung forest. Korea Forest Research Institute, 92 pages.
- Li X., D. Hao, and Y. Huang. 2011. Ant species diversity at piedmont of Zijin Mountain in Nanjing. Journal of Nanjing Forestry University ( Natural Science Edition) 35(5): 55-58.
- Li Z.h. 2006. List of Chinese Insects. Volume 4. Sun Yat-sen University Press
- Lyu D. 2008. Taxonomic study on the Poneromorph subfamilies group (Hymenoptera: Formicidae) in Korea. Korean J. Appl. Entomol. 47(4): 315-331.
- Lyu D. P. 2013. Distribution of Ants(Hymenoptera: Formicidae) by Vegetation in Mt. Gariwangsan from Korea. Korean J. Environ. Ecol. 27(2): 204-208.
- Maeto K. and S. Sato. 2004. Impacts of forestry on ant species richness and composition in warm-temperate forests of Japan. Forest Ecology and Management 187: 213223.
- Manabe K. 1994. Ants of the shrine forest in Fukuoka Prefecture (First report; Ants of lowland). Ari 17: 6.
- Manabe K. 1994. Ants of the shrine forest in Fukuoka Prefecture (first report; ants of lowland). Ari 18: 6.
- Masuko K. 1982. A data on the myrmecofauna of Miyake Island. Ari 10: 1-2.
- Masuko K. 1986. A list of ants collected at Akiyoshi-dai (Yamaguchi-ken) by the members of the Myrmecologists Society (Japan) in 1985. Ari 14:5.
- Masuko, K. 2010. Nest density and distribution of subterranean ants in an evergreen broadleaf forest in Japan with special reference to Amblyopone silvestrii. Entomological Science 13:193
- Matsumura S. and Yamane Sk. 2012. Species composition and dominant species of ants in Jigenji Park, Kagoshima City, Japan. Nature of Kagoshima 38: 99107
- Matsumura S., and S. Yamane. 2012. Species composition and dominant species of ants in Jigenji Park, Kagoshima City, Japan. Nature of Kagoshima 38: 99-107.
- Menozzi C. 1940. Contribution à la faune myrmécologique du Japon. Mushi. 13: 11-12.
- Minato M., T. Kameyama, F. Ito, and T. Itino. 1996. A preliminary report of ant fauna in Gagawa Prefecture. Ari 20: 9-13.
- Miyama D., S. Yamane, T. Hishida, T. Kyono, T. Saito, T. Kuwahara, and N. Inoue. 2007. Ant Fauna in a Coppice Area around Shishitsuka-Ohike in Tsuchiura, Central Japan (Hymenoptera, Formicidae). Bulletin of Ibaraki Nature Museum 10: 1-10.
- Morisita, M. 1941. Notes on Camponotus herculeanus subsp. vagus var. yessensis Teranishi (Hymenoptera, Formicidae). [In Japanese.]. Mushi 13:95. [1941-02-28] PDF 127445
- Ochi K. 1983. Distribution pattern of ants in pine stands, with special reference to Monomorium nipponense Wheeler (Hymenoptera: Formicidae). Gensei 44:1-6.
- Ogata K. 1981. The ant fauna of the Goto islands, Natural history of the Goto? Islands, Japan : Iki Tsushima to no taihi (Danjo Gunto? Ko?rai Sone o fukumu Japan: 347-351.
- Ogata K. 2005. Asian ant inventory and international networks. Report on Insect inventory Project in Tropic Asia TAIIV: 145-170.
- Ogata. K., Touyama, Y. and Choi, B. M. 1994. Ant fauna of Hiroshima Prefecture, Japan. ARI Reports of the Myrmecologists Society (Japan) 18: 18-25
- Ohkawara K., and Y. Fukushima. 1998. A Preliminary report of ant fauna in Ishikawa Prefecture, Central Japan. Ari 22: 6-9.
- Okamoto H. 1972. Ants from Shikoku, Japan (7). Gensei 23:11-14.
- Onoyama K. 1976. A premilinary study on the ant fauna of Okinawa-ken, with taxonomic notes (Japan; Hymenoptera: Formicidae). Ecol. Stud. Nat. Cons. Ryukyu Isl. II: 121-141.
- Our results Winkler 2009
- Park S. H., S. Hosoishi, K. Ogata, and Y. Kuboki. 2014. Clustering of ant communities and indicator species analysis using self-organizing maps. Comptes Rendus Biologies http://dx.doi.org/10.1016/j.crvi.2014.07.003
- Park S.J., K.G. Kim, J.H. Kim, and B.J. Kim. 1998. Ants from the Seoraksan National Park. The Korean Journal of Systematic Zoology 14(4): 429-439.
- Park S.J., and B.J. Kim. 2002. Faunal comparison of ants among Cheongsando and other islands of South Sea in Korea. Korean Journal of Entomology 32(1): 7-12.
- Park, Seong, Joon and Byung, and Kim, Jin. 2002. Faunal Comparison of Ants among Cheongsando and Other Islands of South Sea in Korea. Korean Jornal of Entomology. 32(1):7-12.
- Radchenko, A. 2005. Monographic revision of the ants (Hymenoptera: Formicidae) of North Korea. Annales Zoologici (Warsaw) 55: 127-221.
- Radchenko, A. 2005. Monographic revision of the ants (Hymenoptera, Formicidae) of North Korea. Annales Zoologici 55(2): 127-221.
- Sakai H. 2002. Reproductive flight season of Japanese ants. Ari 26: 33-39.
- Santschi F. 1925. Contribution à la faune myrmécologique de la Chine. Bulletin de la Société Vaudoise des Sciences Naturelles 56: 81-96.
- Santschi F. 1937. Fourmis du Japon et de Formose. Bulletin et Annales de la Société Entomologique de Belgique. 77: 361-388.
- Sato T., N. Tsurusaki, K. Hamaguchi, and K. Kinomura. 2010. Ant fauna of Tottori prefecture, Honshu, Japan. Bulletin of the Tottori Prefectural Museum 47: 27-44.
- Shimana Y., and S. Yamane. 2009. Geogrpahical distribution of Technomyrmex brunneus Forel (Hymenoptera, Formicidae) in the western part of the mainland of Kagoshima, South Kyushu, Japan. Ari 32: 9-19.
- Shimono A., and S. Yamane. 2003. Ant species diversity on Okinoerabu-jima, the Ryukyus, southern Japan. For the Establishment of Remote Islands Study (Kagoshima Univ.) 3: 11-29.
- Shindo, M. 1979. Ants of the Bonin Islands. Konshu to shizen 14(10): 24-28.
- Skarbek C. J., M. Noack, H. Bruelheide, W. Hardtle, G. von Oheimb, T. Scholten, S. Seitz, M. Staab. 2019. A tale of scale: plot but not neighbourhood tree diversity increases leaf litter ant diversity. Journal of Animal Ecology DOI: 10.1111/1365-2656.13115
- Sonthichai S., N. Gavinjan, S. Suwannaratana, and W. Jaitrong. 2006. A comparison of ant populations in restored forest of different ages and adjacent natural vegetation in Northern Thailand. Kasetsart J. (Nat. Sci.) 40: 882-889.
- Staab M., A. Schuldt, T. Assmann, H. Bruelheide, and A.M. Klein. 2014. Ant community structure during forest succession in a subtropical forest in South-East China. Acta Oecologia 61: 32-40.
- Sunamura E., K. Nishisue, M. Terayama, and S. Tatsuki. 2007. Invasion of Four Argentine Ant Supercolonies into Kobe Port, Japan: Their Distributions and Effects on Indigenous Ants (Hymenoptera: Formicidae). Sociobiology 50: 659-674.
- Tang J., Li S., Huang E., Zhang B. and Chen Y. 1995. Hymenoptera: Formicidae (1). Economic Insect Fauna of China 47: 1-133.
- Taniwaki T., H. Kuno, and H. Hosoda. 2005. Annual changes of community structure of ground insects at the isolated small stands in suburbs of Tokyo. Japanese Society of Revegetation Technology 30(3): 552-560.
- Taylor R. W. 1987. A checklist of the ants of Australia, New Caledonia and New Zealand (Hymenoptera: Formicidae). CSIRO (Commonwealth Scientific and Industrial Research Organization) Division of Entomology Report 41: 1-92.
- Terayama M. 1977. Checklist of the known ants of Saitama Prefecture. Insects and nature 12(4): 26-27
- Terayama M. 1981. Distribution of ants of the Nansei Archipelago. (I) Ants in the Amami Islands. Nature and Insects 16(8): 34-36.
- Terayama M. 1983. Kagoshima-ken-hondo no ari. Kanagawa-chucho (Journal of the Kanagawa Entomologists Association): 13-24.
- Terayama M. 1992. Structure of ant communities in East Asia. A. Regional differences and species richness. Bulletin of the Bio-geographical Society of Japan 47: 1-31.
- Terayama M. 1992. Structure of ant communities in east Asia. 1. Regional differences and species richness. Bull. Biogeogr. Soc. Japan 47(1): 1-31.
- Terayama M. 2000. Ants from the Imperial Palace, Tokyo. Mem. Natn. Mus. Tokyo 36: 361-368.
- Terayama M. 2001. Ants of the Institute for Nature study in Minato-ku, Tokyo: species composition, relative nest abundance, and nest density. Rept. Inst. Nat. Stu. 33: 289-300.
- Terayama M. 2009. A synopsis of the family Formicidae of Taiwan (Insecta: Hymenoptera). Research Bulletin of Kanto Gakuen University. Liberal Arts 17:81-266.
- Terayama M. and E. Hasegawa. 1991. Ant fauna of the Ogasawara islands. OgasawaraKenkyuNenpo 15: 4051.
- Terayama M., K. Ogata, and B.M. Choi. 1994. Distribution records of ants in 47 prefectures of Japan. Ari (report of the Myrmecologists Society of Japan) 18: 5-17.
- Terayama M., Y. Tanaka, and S. Tatsuki. 2006. Effects of the invasive ants Linepithema humile (Hymenoptera: Formicidae) on native ant and homopterous insect communities in Japan. Ari 28: 13-27.
- Terayama M., and K. Murata. 1987. Relation between ant communities and vegetations of Toshima island, the Izu Islands. Bull. Biogeogr. Soc. Japan 42(9): 57-63.
- Terayama M., and K. Murata. 1990. Effects of area and fragmentation of forests for nature conservation: Analysis by ant communities. Bull. Biogeogr. Soc. Japan 45(2): 11-17.
- Terayama M., and S. Kubota. 1994. Ants from Aogashima Island. Ari 17: 11.
- Terayama M., and S. Kubota. 2002. Ants of Tokyo, Japan. ARI 26: 1-32.
- Terayama M., and S. Yamane. 1984. Ants of Yaku-shima Island, the northern Ryukyus, with reference to their altitudinal distribution (Insecta: Hymenoptera). Cons. Rep. Yaku-shima Wildness Area, Kyushu, Japan, pp. 643-667. Nat. Cons. Bureau, Env. Agency, Japan.
- Terayama, M. 2009. A synopsis of the family Formicidae of Taiwan (Insecta; Hymenoptera). The Research Bulletin of Kanto Gakuen University 17: 81-266.
- Terayama. M. and Inoue. N. 1988. Ants collected by the members of the Soil Zoological Expedition to Taiwan. ARI Reports of the Myrmecologists Society (Japan) 18: 25-28
- Teruyama. M. 1988. Ant fauna of Saitama Prefecture, Japan. ARI Reports of the Myrmecologists Society (Japan) 16: 4-13
- Touyama Y. 1996. Myrmecofaunal change under fire disturbance. Edaphologia 56: 25-30.
- Touyama Y. 2000. Estimating species richness: an application of the time unit sampling method to a myrmecofaunal survey. Jpn. J. Enviro. Entomol. Zool. 11: 51-60.
- Watanasit S., and T. Nhu-eard. 2011. Diversity of ants (Hymenoptera: Formicidae) in two rubber plantations in Songkhla Province, Soutern Thailand. Songklanakarin J. Sci. Technol. 33(2): 151-161.
- Wheeler W. M. 1906. The ants of Japan. Bulletin of the American Museum of Natural History 22: 301-328.
- Wheeler W. M. 1921. Chinese ants. Bulletin of the Museum of Comparative Zoology 64: 529-547.
- Wheeler W. M. 1927. Ants collected by Professor F. Silvestri in Indochina. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 20: 83-106.
- Wheeler W. M. 1928. Ants collected by Professor F. Silvestri in China. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 22: 3-38.
- Wheeler W. M. 1928. Ants collected by Professor F. Silvestri in Japan and Korea. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 22: 96-125.
- Wheeler W. M. 1930. A list of the known Chinese ants. Peking Natural History Bulletin 5: 53-81.
- Yamane S. 2016. How many species of Ants in Amami Islands? (in Japanese). Part 2, chapter 1 in How many species of Ants in Amami Islands? Pp. 92-132.
- Yamane S. 2019. Seasonal change in the foraging activity of ants in a residential area of mainland Kagoshima, Southwest Japan (Insecta, Hymenoptera, Formicidae). Nature of Kagoshima 45: 361–366.
- Yamane S. 2019. Seasonal change in the foraging activity of ants in a residential area of mainland Kagoshima, Southwest Japan (Insecta, Hymenoptera, Formicidae). Nature of Kagoshima 45: 361–366.
- Yamane S., S. Ikudome, and M. Terayama. 1999. Identification guide to the Aculeata of the Nansei Islands, Japan. Sapporo: Hokkaido University Press, xii + 831 pp. pp, 138-317.
- Yamane S., Y. Harada and M. Yano. 1985. Ant fauna of Tanega-shima Island, the northern Ryukyus (Hymenoptera, Formicidae). Mem. Kagoshima Univ. Res. Center S. Pac. 6(1): 166-173.
- Yamane S., Y. Harada, and K. Eguchi. 2013. Classification and ecology of ants. Natural history of ants in Southern Kyushu. 200 pages
- Yamane S., and S. Ikudome. 2008. Ants, wasps and bees of Kuro-shima Northern Ryukyus, Japan (Hymenoptera, Aculeata). Bull. Inst. Minami-Kyushu Reg. Sci. (Kagoshima Women's Jr. Coll.) 24: 1-9.
- Yamane S.; Bui T. V.; Ogata K.; Okido H.; Eguchi K. 2002. Ant fauna of Cuc Phuong National Park, North Vietnam (Hymenoptera: Formicidae). Bulletin of the Institute of Tropical Agriculture Kyushu University 25: 51-62.
- Yamane S.; Ikudome, S.; Terayama, M. 1999. Identification guide to the Aculeata of the Nansei Islands, Japan. Sapporo: Hokkaido University Press, xii + 831 pp. pp138-317.
- Yamane, S.; Iwai, T.; Watanabe, H.; Yamanouchi, Y. 1994. Ant fauna of the Tokara Islands, northern Ryukyus, Japan (Hymenoptera, Formicidae). WWF (Worldwide Fund for Nature) Japan Science Report 2(2):311-327.
- Yamazaki K., and K. Takakura. 2011. Fauna of Mandai-ike Park in Osaka City. Osaka Tatsuwa Department of Research report (?????????) 73: 75-87.
- Yamazaki Y., S. Yamane, T. Hishida, T. Kuwahara, and N. Inoue. 2009. Ant fauna on the grounds of the Kashima-Jingu Shrine, Ibaraki, Central Japan (Hymenoptera, Formicidae). Bull. Ibaraki Nat. Mus. 12: 5-14.
- Yoshimura M. 2009. Impact of secondary forest management on ant assemblage composition in the temperate region in Japan. J. Insect. Conservation 13(5): 563-568.
- Yoshitomi H., and S. Matsuno. 2012. List of species of Hymenoptera and Diptera in Matsuyama City, Ehime Prefecture, Shikoku, Japan. pp. 167-176. In: Committee for Surveys of Natural Environment of Matsuyama City (Chief Editor: Kazuo ISHIKAWA) (ed.) Checklist of the Wild Animals, Fungi, and Plants of Matsuyama City, 2012. Published by the Department of Environment, Matsuyama City, 404 pp.
- Zhang R., X. Zhou, Q. Tang, and S. Zhou. 2016. Morphometrics of thirteen species of the genus Pachycondyla (Hymenoptera: Formicidae) in China. Chinese Journal of Applied Entomology 53(5): 1130-1137.
- Zhang W., and S. Zhou. 2016. An investigation on Formicidae species of Nanling National Park. Journal of Huizhou University 36(3): 27-30.
- Zhou S.-Y. 2001. Ants of Guangxi. Guangxi Normal University Press, Guilin, China, Guilin, China. 255 pp.
- Pages using DynamicPageList3 parser function
- Common Name
- Highly invasive
- Facultatively polygynous
- Photo Gallery
- Need species key
- North temperate
- North subtropical
- Tropical
- Nesting Notes
- FlightMonth
- Cestode Associate
- Host of Raillietina kashiwarensis
- Karyotype
- Species
- Extant species
- Formicidae
- Ponerinae
- Ponerini
- Brachyponera
- Brachyponera chinensis
- Ponerinae species
- Ponerini species
- Brachyponera species
- Ssr