Camponotus terebrans
| Camponotus terebrans | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Formicinae |
| Tribe: | Camponotini |
| Genus: | Camponotus |
| Species: | C. terebrans |
| Binomial name | |
| Camponotus terebrans (Lowne, 1865) | |
| Synonyms | |
| |
Camponotus terebrans is one of the most common ants in sandy soils of southern Australia and is one of the first ant species to colonise disturbed sites. Nests are sometimes located adjacent to the trunks of trees or shrubs with abundant excavated soil deposited around the numerous entrances. In some cases excavations have been observed to apparently damage or kill nearby shrubs. In other cases nests and their entrances are in open areas and lack mounds. Colonies may be very large and sometimes have "highways" leading to trees and other colonies. This species is often found in association with Ogyris spp. butterflies (Braby 2000). Its castes show wide variation in size, colour, pilosity and profile.
Photo Gallery
Identification
Erect hairs present on scapes and tibiae. Metanotal groove weakly developed and essentially absent. Propodeum with 10 to 25 erect hairs. Pubescence on head and gaster sparse, with individual hairs generally non-overlapping or at most only slightly overlapping. In profile, dorsum of petiolar node angular in both minor and major workers. These characters will separate this taxon from close relatives, especially the morphologically similar Camponotus gouldianus. (Shattuck & McArthur, 2002)
Camponotus terebrans is clearly distinguished from all other ants by the following characters (McArthur, Adams & Shattuck, 1997):
- As a formicine, the acidopore in lateral view is tapering to the orifice, which is fringed with short setae.
- As a member of the genus Camponotus, the antennae are inserted in the head capsule distant from the clypeus by a distance greater than the diameter of the antennal fossae.
- As a member of the C. wiederkehri group, it displays, in lateral and ventral view, J-shaped setae attached to ventral mouthparts usually 4–8 in number and about 0.5 mm long. It could be confused with Melophorus spp., which possess superficially similar J-shaped setae, but in Melophorus the antennae are inserted close to the clypeus.
- The node in lateral view has a summit that is angular or sharp and never smoothly convex.
- Tibiae are pilose, and the longest setae display an uneven inclination ranging from 30° to 70° and are separated by a distance that is equal to or greater than their length, with shorter, more adpressed setae, more closely spaced.
- Antennal scapes are pilose, the longest setae are near erect and sparse along the length of the scape, and the shorter setae are more adpressed and denser.
Keys including this Species
- Key to Australian Camponotus majors of the southwestern Botanical Province
- Key to Australian Camponotus minors of the southwestern Botanical Province
- Key to Australian Camponotus species
Distribution
Latitudinal Distribution Pattern
Latitudinal Range: -29.88333333° to -35.95°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Australasian Region: Australia (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
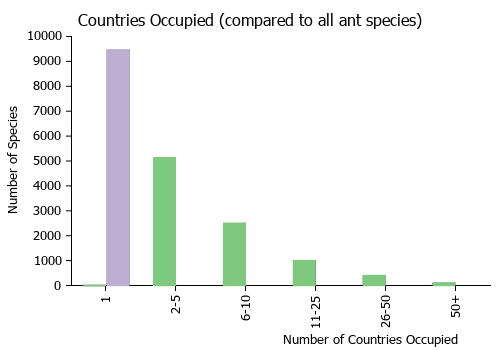
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
From McArthur, Adams & Shattuck, 1997:
Camponotus terebrans is confined to sandy soils. It can show a very patchy distribution in the Gawler Ranges and Sturts Stony Desert, where patches of sand may be only a few hectares. These sandy areas are generally dominated by C. terebrans while the surrounding heavier soils are populated by a different assemblage of ants. For example, at Scrubby Peak (32°37"S, 135°11"E) in the Gawler Ranges, South Australia, in a deposit of sand, C. terebrans was collected, whereas in the surrounding heavier soil, Camponotus gouldianus, Camponotus loweryi, Camponotus wiederkehri and Camponotus consobrinus were collected.
The species is notable for its ability to quickly colonise disturbed habitats. For example, in Helm’s Arboretum near Esperance, Western Australia (33°34"S, 121°53"E), C. terebrans was observed nesting in the sandy soil that had been raised up by a grader during the construction of a track across a swamp. At Mount Hope, South Australia (37°29"S, 140°10"E), C. terebrans was one of the first ants to colonise a sandy site after it was stabilised in 1948 following 50 years of devastation by rabbits, by which time there was no vegetation left and most of the sandy topsoil had blown away. Within a year or two of stabilisation, grasses and trees returned, as did C. terebrans to become the dominant ant. The first colonies were established beneath stones and stumps, but as Allocasuarina stricta (Macklin) L. Johnson and Melaleuca lanceolata Otto germinated and increased in size, the ants excavated tunnels close to the trunks to galleries below. Occasionally, trees with a trunk diameter of up to 100 mm could be found with all soil for about 1 cm surrounding the bark excavated for some depth, causing the tree to be loose in the soil and sometimes causing its death. Similarly loosening soil around shrubs occurred in a garden at Kandara, near Furner.
A.J. McArthur has observed aggressive behaviour by C. terebrans towards two other species. At Mount Hope in 1948, a colony of Iridomyrmex purpureus occupied the heavier ‘terra rosa’ soil on the periphery of the sandy soil, but by 1958 the species had vanished, presumably because of competition with C. terebrans. To this day, C. consobrinus still occupies portions of the adjoining ‘terra rosa’ soil surrounding the C. terebrans territory. Fighting between C. terebrans and C. consobrinus has been observed at the boundary in summer early in the morning.
A.J. McArthur has also observed C. terebrans in what appears to be a dominant role at a sand quarry in Lorne Forest Park, Otway Ranges, Victoria (38°27"S, 143°58"E), where nests of ants of the Myrmecia pilosula species-group are common. These nests are mounds constructed from soil and twigs close to trunks of low bushes. Some were occupied by ants of the M. pilosula species-group while others were occupied by C. terebrans; it appears that C. terebrans has taken over nests originally constructed by the former.
Camponotus terebrans also appears to be the dominant ant in the bed of Strzelecki Creek near Yaningurie Waterhole (28°58"S, 140°06"E), South Australia, where its behaviour is spectacular. Here, the ant was observed to construct long underground tunnels a centimetre or two below the surface of the sandy soil. By scraping the soil away from the top of these tunnels, C. terebrans was revealed running to and fro. The tunnels are prominent. They are marked by long narrow semicircular mounds of soil about 0.5 cm tall on the surface of the creek bed and are often more than 50 m long, connecting colonies near the centre of the creek bed to large Eucalyptus trees on the bank. In July 1989, about 100 of these tunnels were obvious in the creek bed in a space of 5 km downstream from Yaningurie Waterhole, but in July 1994, only three such tunnels could be found.
Foraging Behaviour
An extensive collection of foraging C. terebrans workers was undertaken at Mount Hope, South Australia. This collection demonstrates that only the smallest workers venture far from the nest. Foragers were collected at night by beating a Banksia marginata Cav. shrub growing about 20 m from a nest. A random sample of workers was then collected from this nest. Only the smallest workers were found foraging on the Banksia shrub while both small and large workers were found in the nest. This suggests that major and medium workers of C. terebrans do not travel far from the nest while minor workers can forage much more broadly. Where C. terebrans is densely populated, such as at Mount Hope and Stockyard Plain, diurnal foragers have been observed, although nocturnal foraging is more general. Nests of Camponotus aurocinctus (Smith) have been observed within a few metres of nests of C. terebrans in sandy soil at Stockyard Plain and at Danggali Conservation Park.
Associations with Other Invertebrates
Coleoptera: Tenebrionidae. There is a relationship between C. terebrans and the tenebrionid beetle Camponotiphilus fimbricollis Lea, involving co-habitation (Mathews 1993). C. terebrans and Camponotiphilus fimbricollis have been taken from the same nest at Beverley, Western Australia (32°07"S, 116°56"E), by F. H. duBoulay (SAMA) and at Hattah Lakes, Victoria (34°45"S, 142°20"E) (NMVA).
Lepidoptera. Camponotus terebrans is believed to have a mutualistic relationship with a butterfly (Ogyris spp.). In these cases, the entrance to the nest was generally close to a food plant of Ogyris, such as Choretrum glomeratum R. Br.
Castes
Worker
Images from AntWeb
   
| |
| Worker. Specimen code casent0280206. Photographer Will Ericson, uploaded by California Academy of Sciences. | Owned by PSWC, Philip S. Ward Collection. |
   
| |
| Holotype of Formica testaceipes. Worker (major/soldier). Specimen code casent0903534. Photographer Z. Lieberman, uploaded by California Academy of Sciences. | Owned by NHMUK, London, UK. |
   
| |
| Syntype of Camponotus terebrans. Worker (major/soldier). Specimen code casent0903533. Photographer Z. Lieberman, uploaded by California Academy of Sciences. | Owned by NHMUK, London, UK. |
   
| |
| Syntype of Camponotus latrunculus victoriensis. Worker (major/soldier). Specimen code casent0911963. Photographer Z. Lieberman, uploaded by California Academy of Sciences. | Owned by NHMB, Basel, Switzerland. |
   
| |
| Syntype of Camponotus latrunculus victoriensis. Worker. Specimen code casent0911964. Photographer Z. Lieberman, uploaded by California Academy of Sciences. | Owned by NHMB, Basel, Switzerland. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- testaceipes. Formica testaceipes Smith, F. 1858b: 39 (w.) AUSTRALIA (Western Australia).
- [Junior primary homonym of Formica testaceipes Leach, 1825: 290 (Emery, 1921a: 24).]
- Mayr, 1862: 662 (q.); Forel, 1902h: 494 (s.m.).
- Combination in Camponotus: Mayr, 1862: 662;
- combination in C. (Myrmophyma): Forel, 1914a: 269;
- combination in C. (Tanaemyrmex): Emery, 1925b: 102.
- Status as species: Mayr, 1862: 662 (redescription); Mayr, 1863: 401; Roger, 1863b: 4; Mayr, 1876: 60 (in key); Emery, 1887a: 211; Dalla Torre, 1893: 254; Emery, 1896d: 372; Forel, 1902h: 494; Forel, 1907h: 301; Wheeler, W.M. 1909a: 29; Forel, 1915b: 100; Emery, 1925b: 102; Wheeler, W.M. 1934d: 159; Brown, 1956a: 39; Taylor & Brown, 1985: 120.
- Replacement name: Formica terebrans Lowne, 1865a: 278.
- [Note: terebrans oldest junior synonym of testaceipes Smith, F. (synonymy by Mayr, 1876: 65); hence terebrans first available replacement name (Bolton, 1995b: 126).]
- terebrans. Formica terebrans Lowne, 1865a: 278 (s.w.q.) AUSTRALIA (New South Wales).
- Replacement name for Formica testaceipes Smith, F. 1858b: 39. [Junior primary homonym of Formica testaceipes Leach, 1825: 290 (Emery, 1921a: 24).]
- [Note: terebrans junior synonym of testaceipes Smith, F. (synonymy by Mayr, 1876: 65); hence terebrans first available replacement name (Bolton, 1995b: 126).]
- Junior synonym of testaceipes: Mayr, 1876: 65; Dalla Torre, 1893: 254; Emery, 1896d: 372 (in list); Emery, 1925b: 102; Taylor & Brown, 1985: 120; Taylor, 1987a: 15.
- Status as species: Bolton, 1995b: 126; McArthur, Adams & Shattuck, 1998: 587 (redescription); Shattuck & McArthur, 2002: 82 (redescription); McArthur, 2007a: 310; Heterick, 2009: 61; McArthur, 2010: 48; McArthur, 2014: 96.
- Senior synonym of myoporus: McArthur, Adams & Shattuck, 1998: 587; Shattuck & McArthur, 2002: 82; McArthur, 2007a: 294; McArthur, 2010: 48.
- Senior synonym of victoriensis: McArthur, Adams & Shattuck, 1998: 587; Shattuck & McArthur, 2002: 82; McArthur, 2007a: 294; McArthur, 2010: 48.
- myoporus. Camponotus (Tanaemyrmex) myoporus Clark, 1938: 379, figs. 20-22 (s.w.) AUSTRALIA (South Australia: Reevesby I.).
- Status as species: Taylor & Brown, 1985: 117; Taylor, 1987a: 13; Bolton, 1995b: 112.
- Junior synonym of terebrans: McArthur, Adams & Shattuck, 1998: 587; Shattuck & McArthur, 2002: 82; McArthur, 2007a: 294; McArthur, 2010: 48.
- victoriensis. Camponotus latrunculus var. victoriensis Santschi, 1928e: 479 (s.w.m.) AUSTRALIA (Victoria).
- Subspecies of latrunculus: Taylor & Brown, 1985: 116; Taylor, 1987a: 13; Bolton, 1995b: 129.
- Junior synonym of terebrans: McArthur, Adams & Shattuck, 1998: 587; Shattuck & McArthur, 2002: 82; McArthur, 2007a: 294; McArthur, 2010: 48.
Type Material
- Camponotus (Myrmoturba) latrunculus victoriensis Santschi, 1928: Syntype, 1 major, 1 medium and 1 minor worker, Belgrave, Victoria, Australia, Barrett, Naturhistorisches Museum, Basel.
- Camponotus (Myrmoturba) latrunculus victoriensis Santschi, 1928: Syntype, 1 major and 1 minor worker, 1 male, Elsternwick, Victoria, Australia, Barrett, Naturhistorisches Museum, Basel.
- Camponotus (Tanaemyrmex) myoporus Clark, 1938: Syntype, 3 workers, Reevesby Island, South Australia, Australia, Mahony,D.J., McCoy Soc. Expedition, ANIC32-017680, Australian National Insect Collection.
- Camponotus (Tanaemyrmex) myoporus Clark, 1938: Syntype, 1 major and 1 minor worker, Reevesby Island, South Australia, Australia, J. Clark, Museum Victoria, Melbourne.
- Formica terebrans Lowne, 1865: Syntype, 2 minor and 1 medium worker, Sydney (as Sidney), New South Wales, Australia, Lowne, F. Smith Coll 79/22, The Natural History Museum.
- Formica testaceipes Smith, 1858: Holotype, worker, King George Sound (as King George's Sound), Western Australia, Australia, Drawer 11-486, Type/Sm. BH Type 11-619, The Natural History Museum.
Description
From Shattuck & McArthur (2002):
Major worker
Medial section of anterior clypeal margin straight, projecting anteriorly with rectangular lateral corners, crenulate; carina indistinct. Pronotum and mesonotum weakly convex; metanotum distinct as two parallel, transverse grooves; dorsal surface of propodeum straight, angle well rounded, posterior face mostly straight, length of dorsal and declining faces about equal. Anterior face of petiolar node convex, summit sharp, posterior face mostly straight. Entire body with plentiful long erect setae tending to suberect on tibiae and scape, absent from funiculi. Head red-brown to black, funiculi lighter, mesosoma and node yellow to brown, gaster darker than mesosoma, legs lighter.
Minor worker
Anterior clypeal margin with median section convex and strongly projecting, carina distinct. Pronotum and mesonotum mostly weakly convex; the smallest workers without a metanotal groove; dorsal propodeal surface straight, angle well rounded, posterior face straight, ratio dorsum to declivity exceeds 2 in smallest workers (Fig. 47). Anterior and posterior faces of petiolar node generally parallel, summit bluntly convex. Entire body with plentiful long and short erect setae tending to suberect on tibiae and scape, absent from funiculi. Head brown, funiculi lighter, mesosoma and node yellow to brown, gaster darker than mesosoma, limbs lighter.
Measurements
Workers (n=20). Cl 0.85 (minors) - 1.11 (majors); HL 1.36mm - 3.28mm; HW 1.15mm - 3.64mm; ML 2.07mm - 3.64mm; MTL 1.56mm - 2.39mm; PnW 0.91mm - 2.02mm; SI 0.66 (majors) - 1.54 (minors); SL 1.77mm - 2.39mm.
From McArthur, Adams & Shattuck, 1997:
Major worker. Lateral view. Head dark brown to red-brown, sides finely reticulate, glossy. Scape dark brown to black. Funiculus dark brown to yellow-brown. Vertex with 6–10 long and short setae. Gula with 10–20 setae. Dorsal view. Head sides mostly strongly convex then tapering to the front. Vertex straight, concave in some other views. Setae on scape (near centre) distinct, sparse, sub-erect to erect, pubescence adpressed to decumbent, distinct, spaced less than their length apart. Frontal carinae, anterior quarter converging then diverging, next quarter straight diverging, posterior half parallel then diverging. Frontal area, indistinct, small, elongated diamond shape. Head width reaches maximum near a line through eye centre. Six teeth on the masticatory border plus a small tooth on the basal border near the angle. Clypeus anterior margin, lateral quarters transverse straight, median half strongly projecting to the front bounded by 90° angles, with crenulations. Integument of clypeus finely reticulate, sparsely and feebly punctate, glossy. Pubescence on clypeus adpressed, indistinct, spaced much greater than their length apart. Carina indistinct. Lateral view. Pronotum brown to yellow, sometimes mottled, from an anterior narrow flat plate follows a gentle concavity then a gentle convexity, with 10–20 setae. Mesonotum brown to yellow, sometimes mottled, flatly convex, with 10–20 setae. Metanotum distinct, usually straight although sometimes convex, sometimes sloping down towards the node, usually glabrous, separated from the propodeum by a groove usually shorter than a quarter of the length of the metanotum, rarely half as long. Propodeum brown to yellow, with 10–20 setae, dorsum either straight or feebly convex, angle rounded and often indistinct, generally about 130° but sometimes a little sharper in largest major workers, upper three-quarters of declivity straight, length of dorsal and declining faces about equal. Integument near propodeal spiracle finely reticulate and usually glabrous, pubescence much denser below than above, spiracle elongated and placed on the side well forward of declivity. Node brown to yellow, anterior face convex, summit sharp, posterior face mostly straight, pubescence adpressed, usually sparse. Gaster darker than mesosoma, usually black or dark brown, integument of first gastric tergite microscopically finely striate, glossy. Fore coxa always lighter than pronotum, yellowish rarely whitish. Fore femur always lighter than pronotum, yellowish rarely whitish. Fore tibia red-yellow to red-brown. Fore tarsus red-yellow to red-brown. Mid-tibiae with outer setae 0.2 mm long, spaced greater than their length apart, inclined about 60°, pubescence adpressed distinct, 9 or 10 bristles in each of 2 rows on inner surface, often hard to isolate from surrounding setae. Front or rear view. Node summit bidentate in largest major workers, otherwise convex with 6–10 long setae.
Minor worker. Lateral view. Head black or brown to red-brown with sides finely reticulate, glossy. Scape from dark brown to black. Funiculus from dark brown to yellow-brown. Vertex with 10–50 long and short setae. Gula with 10–20 setae. Dorsal view. Head sides parallel and feebly convex, vertex strongly convex. Scape (near centre) with distinct sparse sub-erect to erect setae, pubescence adpressed to decumbent, spaced less than their length apart, distinct. Frontal carinae overall nearly parallel, anterior quarter feebly converging, centre half straight, feebly diverging, posterior quarter feebly converging. Frontal area distinct, clypeal margin convex. Head width reaches maximum near eye centre. Six teeth on the masticatory border plus a small tooth on basal border near the angle. Clypeus anterior margin lateral sixths straight transverse, median two-thirds evenly convex, strongly projecting. Clypeus finely reticulate, glossy. Pubescence on clypeus decumbent, coarse, sparse. Carina distinct. Lateral view. Pronotum brown to yellow, from an anterior flat plate with short erect setae there is a short step to the evenly flatly convex dorsum, with 10–30 setae plus decumbent and adpressed pubescence. Mesonotum brown to yellow, flatly convex, with 10–20 setae. Metanotum obsolete. Propodeum brown to yellow, with 10–20 setae, dorsum straight, angle about 160° rounded, declivity upper three-quarters straight, ratio dorsum to declivity exceeds 2 in smallest minors. Integument near propodeal spiracle finely reticulate, adpressed pubescence usually partially hiding integument, spiracle elongated and well forward of declivity. Node brown to yellow, anterior face convex, summit angular, posterior face lower three-quarters straight, upper quarter convex, pubescence adpressed, usually sparse. Gaster darker than mesosoma, usually black or dark brown, integument of first gastric tergite microscopically finely striate, glossy. Fore coxa usually lighter than pronotum, yellowish rarely whitish. Fore femur usually lighter than pronotum, yellowish rarely whitish. Fore tibia red-yellow to red-brown. Fore tarsus red-yellow to red-brown. Mid tibia with setae on outside 0.2 mm long, spaced greater than their length apart, inclined about 60°, pubescence outside adpressed, distinct, with 2 rows of 9 or 10 bristles on insides. Front or rear view. Node summit strongly convex with 6–10 long setae.
Medium worker. Characters are intermediate between major and minor workers.
Morphological Analysis
Several morphological characters show considerable variation. These include colour, the profiles of the propodeum and metanotum, and the lengths of the eyes and mid-tibiae. Details of these characters are as follows.
The colour of the mesonotum varies from dark in southern and western regions to light in inland areas. This change in colour is gradual and uniform between the extremes of the range, and light and dark individuals have never been found in sympatry. The size of the eyes and the mid-tibia show a distinct north–south clinal pattern similar to that found in body colour. In southern regions the eyes are relatively smaller while in northern areas the eyes are larger and legs are longer. The variation seen in these characters shows gradual and continual change across these areas and is considered clinal.
The variation in the curvature of the metanotum and propodeum is most noticeable in major workers. This surface varies from straight to feebly convex and intra-nest variation can be greater than inter-nest variation. In all major workers the metanotum itself is distinct but variable (particularly when viewed from the side). The metanotum fades as size decreases and is completely absent in minor workers. No geographic patterns were detected in the variation of these characters.
Systematic Implications of the Allozyme Data
The allozyme profiles at 32 loci for the 43 ants, representing 25 nests, were included in the initial screen. Sixteen loci displayed allozyme variation, with a maximum of four allozymes found at each of several loci. Whilst within-nest polymorphism was common, all 68 cases involved the presence of only two allozymes, and in only one of these cases were no heterozygotes detected (Acon-1 for Nest 23). As such, it is appropriate to employ our previous approach of using the nest as the unit of analysis (McArthur and Adams 1996).
Overall, nests show a high degree of genetic similarity across the geographic range sampled, with a maximum of 13%FD found between any two nests, and an average level of divergence of 5.4%FD. Nevertheless, two major groupings are evident, differing at an average of 9.1%FD (equating to approximately three fixed differences). Most significantly, the geographic distribution of the two groups is distinctly non-random, and reflects a simple, underlying geographic pattern. First, there is a ‘southern’ group (Nests 1–8) consisting of all of the Western Australian nests except the two from Kalgoorlie, plus those from Fleurieu Peninsula, South Australia (Nests 21–25). The second group consists of the ‘northern’ nests, namely the two from Kalgoorlie (Nests 9, 10) plus the remaining six northern locations from South Australia (Nests 11–20). An examination indicates that the northern and southern groups can be diagnosed unequivocally by their allozyme profiles at the loci Est-2 and Acon-1 (although only Est-2 is fully diagnostic on its own), with further differentiation within either Western Australia or South Australia apparent at the loci Enol and Est-1.
No other major genetic discontinuities are evident from the allozyme data, with only a single locus per group showing any evidence of regional differentiation within each of the southern and northern groups. For the southern group a comparison of the Western Australian with the South Australian nests reveals a near-fixed difference at the locus Enol. The northern group also shows within-group divergence at one locus, with the Kalgoorlie nests possessing a near fixed difference at Est-1 from those in South Australia. The genetic divergence at these two loci is responsible for the primary dichotomies found within each group. Thus, the genetic divergence within each group across a geographic range of more than 1000 km is clearly less than that displayed between the northern and southern groups, which were sampled to within 200 km of one another (e.g. Esperance and Kalgoorlie in Western Australia, Cox Scrub and Calperum in South Australia).
In an attempt to resolve the ambiguous pattern of genetic diversity observed in C. terebrans, additional nests were sampled throughout eastern South Australia from the Riverland region southwards. All available blocks of sandy habitat were visited in an effort to locate additional nests. In this manner, nine nests from a further seven sites were sampled, with these additional ants being analysed only for the enzymes ACON, EST, ENOL, GPI, PEPB and TPI, the most informative genetic markers from the initial screen.
Even though the formerly diagnostic locus Est-1 now displays a few heterozygotes, once again the overall allozyme profiles at Acon-1, Est-1 and Est-2 reveal a distinction between the two northern-type nests (Nests 33 and 34 from Stockyard Plain) and all others to the south, including Nest 32 from Billiat Conservation Park, the first patch of sandy terrain to the south. The maintenance of the dichotomy between the northern and southern groups is better demonstrated by a PCoA analysis on the Rogers’ genetic-distance matrix of pairwise comparisons of all of the 24 nests sampled in South Australia (data from the nine loci run for all nests). The distinction between the northern and southern groups is clearly shown, with all nests falling within either one or the other of the two clusters. Thus there is reasonable genetic integrity within each of the two groups across distances of roughly 300 km in the case of the southern group (e.g. Billiat to Beachport) and 600 km for the northern nests (e.g. Mildura to Lake Eyre), albeit based largely on the results of three loci, none of which is fully diagnostic when applied individually.
Integration of the Morphological and Allozyme Analyses
The allozyme data suggest the presence of two genetic groups within C. terebrans, broadly corresponding to a northern and a southern group. The two groups are genetically similar, with levels of divergence between adjacent regions of 3–6%FD, based mainly on combinations of nearly fixed differences at three loci. Our attempts to find these two forms in sympatry in South Australia were not successful, although they do occur as close as 100 km on adjacent blocks of sandy terrain (Billiat and Stockyard Plain). However, as only a relatively small number of nests was sampled, it may be that more-robust estimates of allele frequency for populations would reveal the presence of all key northern and southern alleles in this geographically intermediate region. We were unable to conduct detailed sampling in Western Australia, where a transect from Esperance to Kalgoorlie would be highly desirable as it would include both forms. This lack of sympatric associations of the genetic types is consistent with morphology, where body colour and eye size show a similar pattern.
While the level of genetic and morphological divergence found between the northern and southern groups of these ants suggests that they may represent distinct taxa, it is not of sufficient magnitude in allopatry to draw the definite conclusion that they represent distinct biological species. For example, it is possible that the two groups represent the extremes of a north–south cline, or that they are formerly disjunct subspecies that may be in the process of merging into a single metapopulation. Further sampling in geographically intermediate regions is needed to resolve this borderline systematic scenario.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Formica terebrans Lowne, B.T. (1865).
Small worker 4 lines long(= 8 mm). Head and abdomen black; antennae, thorax and legs piceous. Head large, broader than the thorax, rounded behind, rufo-piceous anteriorly. Mandibles large, triangular, strongly dentate within and obscurely rufo-piceous. Antennae long and slender. Eyes large, ovate and prominent. Thorax rounded anteriorly; the meso- and metathorax much compressed laterally, with a small raised ocellate spot on each side of the mesothorax. Scale of the peduncle ovate, pointed above. Abdomen ovate, the apical margins of its segments testaceous, thinly covered with pale silky hairs. Large worker 5 lines long, with a very large head.
Female 6 lines long. Black. Wings subhyaline, with fuscous nervures. These insects excavate the hard dead stumps of gum trees (Eucalypti) with complicated galleries. Early in October I found winged females only in a nest; they were apparently hybernating, as they were packed closely in closed galleries, which I cut into by accident whilst searching for wood-boring beetles. A few days after I found swarms of the winged females, clustering about the flowers of Boronias and other Rutaceae, for several days. In December I found numerous colonies of these insects, with abundance of large and small workers, but I sought for the sexes in vain.
Formica testaceipes Smith,F. (1858)
Worker. Length 4 lines (= 8 mm) Shining black: the legs flavo-testaceous, the flagellum testaceous; the posterior angles of the head, two distinct maculae on the prothorax above, and also the disc of the metathorax, ferruginous. The head deeply emarginate behind, very smooth and shining; the anterior margin of the clypeus widely emarginate. Thorax smooth, anteriorly rounded, posteriorly compressed. Abdomen ovate; the scale of the peduncle ovate and notched above.
Hab. Australia (King George's Sound)
References
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Heterick, B.E. 2021. A guide to the ants of Western Australia. Part I: Systematics. Records of the Western Australian Museum, Supplement 86, 1-245 (doi:10.18195/issn.0313-122x.86.2021.001-245).
- Heterick, B.E. 2022. A guide to the ants of Western Australia. Part II: Distribution and biology. Records of the Western Australian Museum, supplement 86: 247-510 (doi:10.18195/issn.0313-122x.86.2022.247-510).
- Lowne, B. T. 1865a. Contributions to the natural history of Australian ants. Entomologist 2: 275-280 (page 278, soldier, worker, queen described)
- Mayr, G. 1876. Die australischen Formiciden. J. Mus. Godeffroy 12: 56-115 (page 65, Junior synonym of testaceipes)
- McArthur, A. J.; Adams, M. 1996. A morphological and molecular revision of the Camponotus nigriceps group (Hymenoptera: Formicidae) from Australia. Invertebr. Taxon. 10: 1-46 (page 41, Type-series of lividipes referred to terebrans-group (as testaceipes-group))
- McArthur, A. J.; Adams, M.; Shattuck, S. O. 1998 [1997]. A morphological and molecular review of Camponotus terebrans (Lowne) (Hymenoptera: Formicidae). Aust. J. Zool. 45: 579-598.
- Shattuck, S. O.; McArthur, A. J. 2002. A taxonomic revision of the Camponotus wiederkehri and perjurus species-groups (Hymenoptera: Formicidae). Trans. R. Soc. S. Aust. 126: 63-90 (page 82, figs. 44-48 major, minor worker described)
References based on Global Ant Biodiversity Informatics
- Clark J. 1938. The Sir Joseph Banks Islands. Reports of the McCoy Society for Field Investigation and Research. Part 10. Formicidae (Hymenoptera). Proceedings of the Royal Society of Victoria (n.s.)50: 356-382.
- Emery, C.. "Catalogo delle formiche esistenti nelle collezioni del Museo Civico di Genova. Parte terza. Formiche della regione Indo-Malese e dell'Australia." Annali del Museo Civico di Storia Naturale Giacomo Doria (Genova) (2) 4, no. 24 (1887): 209-258.
- Forel A. 1893. Nouvelles fourmis d'Australie et des Canaries. Ann. Soc. Entomol. Belg. 37: 454-466.
- McArthur A. 2010. A guide to Camponotus ants of South Australia. Adelaide: South Australian Museum, IV + 121 pp.
- McArthur A. J., M. Adams, and S. O. Shattuck. 1998. A morphological and molecular review of Camponotus terebrans (Lowne) (Hymenoptera: Formicidae). Aust. J. Zool. 45: 579-598.
- McArthur A. J., and R. Leys. 2006. A morphological and molecular study of some species in the Camponotus maculatus group (Hymenoptera: Formicidae) in Australia and Africa, with a description of a new Australian species. Myrmecologische Nachrichten 8: 99-110.
- Santschi F. 1928. Nouvelles fourmis d'Australie. Bulletin de la Société Vaudoise des Sciences Naturelles. 56: 465-483.
- Shattuck S. O., A . J. McArthur. 2002. A taxonomic revision of the Camponotus wiederkehri and perjurus species-groups (Hymenoptera: Formicidae). Transactions of the Royal Society of South Australia 126: 63-90.
- Taylor R. W. 1987. A checklist of the ants of Australia, New Caledonia and New Zealand (Hymenoptera: Formicidae). CSIRO (Commonwealth Scientific and Industrial Research Organization) Division of Entomology Report 41: 1-92.
- Wheeler W. M. 1934. Contributions to the fauna of Rottnest Island, Western Australia. No. IX. The ants. Journal of the Royal Society of Western Australia 20: 137-163.



