Lasius neglectus
| Lasius neglectus | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Formicinae |
| Tribe: | Lasiini |
| Genus: | Lasius |
| Section: | niger clade |
| Species group: | brunneus |
| Species complex: | turcicus |
| Species: | L. neglectus |
| Binomial name | |
| Lasius neglectus Van Loon, Boomsma & Andrasfalvy, 1990 | |
A widespread European species that forms supercolonies and has been spread through human activity. It can be a pest species, entering buildings, disrupting soil in gardens and roadworks and damaging power supplies with its nest construction. Colonies in natural habitats are polygynous but monogynous, has a true nuptial flight and have workers that are aggressive toward conspecific workers from other nests. Supercolonies are only found in the non-native range of this species. The native range of L. neglectus is Central Asia (particularly, Uzbekistan) (Stukalyuk et al., 2020). Pashaei Rad et al. (2018) found this species in Iran on the ground in a moist forest.
| At a Glance | • Supercolonies • Highly invasive |
Identification
Worker: This species belongs in a group of Lasius that lack erect hairs on the scapes and on the extensor profile of hind tibiae. Mandibular dentition is reduced (seven denticles; rarely eight) as compared with Lasius lasioides, Lasius alienus, Lasius psammophilus, Lasius paralienus or Lasius piliferus although this is a difference of statistical character.
Queen: Immediately recognizable among the European Lasius by its comparatively reduced size and proportionately smaller gaster, as compared with the thorax.
Male: The smallest male within the European Lasius (s.str.) species.
Seifert (2020) - Palaearctic Lasius s. str. species belonging to the Lasius turcicus species complex. Lasius turcicus is the sister species of L. neglectus (Steiner et al. 2004). The determination of this species in introduced areas, where no Lasius turcicus or Lasius precursor are present, is usually simple due to the impressive colony structure of L. neglectus. Colonies may initially be confused with Lasius psammophilus and Lasius obscuratus. The latter species differ from L. neglectus by its longer pronotal setae, shorter maxillary palps and higher number of mandibular dents. Separation from L. turcicus and L. precursor is more challenging and requires assessment of complex character combinations. (see Seifert 2020 for details).
Keys including this Species
Distribution
Lasius neglectus is widespread from Canary Islands to Central Asia, inhabiting almost exclusively anthropogenic habitats (cities, towns, villages). Stukalyuk et al. (2020) found numerous populations of this species in Uzbekistan, where it lives in the natural habitats throughout the country except for arid zones — the Kyzylkum desert and the Ustyurt plateau. It inhabits mesophytic, moderately humidified biotopes at altitudes from 91 to 1982m a.s.l., but is also common in urban areas.
Seifert (2020) – A highly invasive species that has spread from its center, which is presumed to be somewhere in Asia Minor. It has been reported from: Tenerife, Iberia, France, Switzerland, Corsica, Italy, S England, Netherlands, Belgium, Germany, Poland, Hungary, Balkans, Ukraine, Cis- and Transcaucasia, Asia Minor, Iran, Uzbekistan, Kyrgyzstan (75°E), Israel. The northernmost known site in Europe by the year 2013 is Rostock (54.1°N). In Asia Minor L. neglectus is most abundant below 1000 m but some populations ascend as high as 1900 m.
This is known mostly from eastern islands, especially from tourist resorts. Confirmed records are from the Aegean Islands, Cyclades, the Dodecanese and from two mainland provinces, Peloponnese and Thrace (Borowiec et al., 2022).
Latitudinal Distribution Pattern
Latitudinal Range: 44.8° to 36.2976°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Palaearctic Region: Andorra, Belgium, Bulgaria, Canary Islands, Croatia, France, Georgia, Germany, Greece, Hungary (type locality), Iberian Peninsula, Iran, Israel, Italy, Kyrgyzstan, Netherlands, Poland, Romania, Russian Federation, Spain, Switzerland, Türkiye, United Kingdom of Great Britain and Northern Ireland, Uzbekistan.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
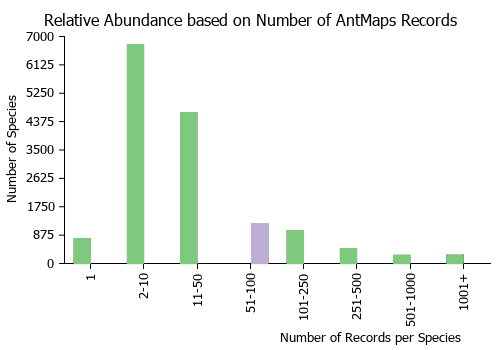
|
Biology
Life cycle
The scarce data noted below probably do not reflect the variation to be found between populations as northern as that from Warsaw (Poland) and those from Iran. As compared with other Lasius species, the sexuals appear earlier in the year: as early as March, within heated buildings in Budapest (Andrásfalvy, in litt.). In three studied populations from NE Spain, activity throughout the year shows remarkably similar duration, beginning in early March until late November, when certain colonies in protected zones are still active.
Daily activity
In two Spanish populations the ants remain active for 24 h/day from May to late September, with temperature controlling the daily cycle.
Foraging
This ant seems to be highly dependent on aphid honeydew. In North-east Spain, during the early season, when leaves are still lacking on deciduous trees or tree aphids are scarce, this ant constructs earth tents over small herbs protecting stem and root aphids. In the full season ants visit aphids on different tree species in huge numbers and in rare instances individuals are seen carrying small prey (collembola, psocoptera). Ants are active throughout the entire day and aphid tending lasts for 24 h/day, from late April to late October, imposing a non-negligible cost on the energetic budget of individual trees. Preliminary quantitative measures indicate that ants can extract a mean of 250 cc honeydew per month on holm oak (Quercus ilex) and as much as 950 cc honeydew per month on poplar trees (Populus nigra). In a recent study (Paris & Espadaler 2009) food collection performed by the invasive ant Lasius neglectus and by the native ant Lasius grandis was compared. The invasive ant collected 2.09 kg of honeydew per tree while the native ant collected 0.82 kg. The aphid Lachnus roboris was visited by both ant species. In holm oaks colonized by L. neglectus, aphid abundance tended to increase and its honeydew production increased twofold. The percentage of untended aphids was lower in holm trees occupied by L. neglectus. Tending ants may also prey on insects: the native ant workers carried more insects than the invasive ant. Both ant species preyed mainly on Psocoptera and the rarely tended aphid, Hoplocallis picta. We conclude that the higher honeydew collection achieved by L. neglectus was the consequence of (1) its greater abundance, which enabled this ant to tend more Lachnus roboris and (2) its greater level of attention towards promoting an increase of honeydew production. Tree species occupied in Spain.
Lasius neglectus can recruit to food finds en masse. Frizzi et al. (2018) studied the mechanisms and nuances of recruitment in this species, including examining how individuals and groups respond to and potentially choose optimal food resources. They found L. neglectus can rapidly detect the most profitable resource and quickly recruit large numbers of conspecifics. Trail marking intensity decreased far less over time when compared to that of Lasius niger. This trait makes the behaviour of L. neglectus similar to that of other invasive species, such as the Pharaoh ant Monomorium pharaonis.
Sexuals physiological condition and behaviour
Nuptial flight seems to be absent. In a single instance alate males and queens were found in a spider net on a house wall (Seifert 2000: 178), although this is not a definite proof of flying behaviour. Except for this case, sexuals have never been detected flying out of the nest. Intranidal mating, thus, is probably the rule (Van Loon et al. 1990; Espadaler & Rey 2001).
Social structure
Depending on the populations, colonies are very difficult to delimit as they may coalesce and integrate a supercolony occupying continuous areas, as large as 17 ha. In urban areas the colonies are considerably split and may ocupy a single tree and up to 3600 ha. Finding many dealate queens (polygyny) in a nest is a key diagnostic characteristic of this species, the single polygynous European Lasius (s.str.). This biological aspect is very probably the best way to identify it, although it is advisable to verify with the morphology. The number of queens depends on colony size. Queen number, estimated by queens found under stones, is about 35500 in the supercolony of Seva. Using soil cores, worker number for that population in May 2002, was estimated as 1.12 x 108 (Espadaler et al. 2004). The species merits the qualification of unicolonial.
Internest and interpopulation relationships show the usual trait already known for unicolonial ants: a reduced level of agressiveness, though some non-native populations show higher levels of aggression in lab tests. Laboratory tests on aggression should be refined to be fully applicable to this light-avoiding ant. Have a look at its less than stressful tactics and compare with the much more aggressive Argentine ant.
Aggression levels
Lasius neglectus is highly aggressive against three native Iberian Lasius species (Lasius grandis, Lasius emarginatus and Lasius cinereus), expressed as a higher attack rate of L. neglectus and behavioural dominance throughout the aggressive encounters. Attacks of L. neglectus were performed fastest and most frequent against L. grandis, and also the highest antennation frequencies were observed in encounters between these two species. This could be due to the largest difference in body size, or due to a greater overlap in ecological niche between L. neglectus and L. grandis compared to the other two native species (Cremer et al. 2006).
Nesting Habits
The areas occupied furnish a wide array of possible nesting sites: under stones, temporal refuges with aphids at the base of herbs, amid rubbish.
Non-native populations of this ant are usually related to human-modified habitats -ranging from purely urban habitats in streets with heavy traffic to city gardens, urban woods, small towns -or semi-urban areas.
Colony expansion
The expansion process of a supercolony in Spain seems to be much helped by the progresive urbanization of lots. This development usually implies the cutting and burning of all natural vegetation but trees. The planting of grass and continuous irrigation of grass that follows favours the establishment of the ants. See also a map showing the area of the Debrecen supercolony (data from 1998, 2000, and 2002). The presence of different ant species' nest entrances was mapped. The expansion of L. neglectus was variable in space and time. It seems that L. neglectus spreads fastest on paths, and does not spread rapidly in shady and cool areas occupied by coniferous bushes. The data suggest that the relative Lasius niger is more impacted by the invasion of L. neglectus than Tetramorium cf. caespitum. Moreover, Liometopum microcephalumand Lasius fuliginosus were able to defend their territory (Tartally 2006).
A recent experimental research by Ugelvig & Cremer (2007), using L. neglectus from four populations (Bellaterra, Jena, Seva, Volterra) is the first demonstration of contact immunity in social Hymenoptera. Social contact with individual workers that were exposed to a fungal parasite (Metarhizium anisopliae var. anisopliae) provided a clear survival benefit to non-treated ants, upon later contact with the same parasite. Behaviour was also affected: brood care was absent in infested ants whereas naive nestmates increased brood-care activities. The collective behavioural and physiological prophyllaxis work to promote the immunity of the society and to counteract the high risk of disease transmission.
Effects on other ants and arthropods in human-influenced habitats
Through their geographical distribution, the distinct populations live in a wide range of conditions, from strictly urban habitats - streets with heavy traffic to semi-urban sites, mildly degraded habitats or seemingly undisturbed localities. A common feature to all such places is the presence of trees, on whose aphid populations the ants depend.
Some of those populations have attained pest status, affecting man or other biological components. Those populations can properly be qualified as invasive (=an agent of change, threatening native biological diversity). Other populations are still merely established and seem to have a more limited expansion as they have yet to be reported as pernicious; this may correspond to the lag phase found in many invaders. Perhaps this is only due to lack of knowledge or, alternatively, climate has, indeed, a limiting effect on the dispersal or expansion processes.
From its description, it is known that in the areas occupied by this species, other surface-foraging ant species have vanished or have very reduced populations. Spatial and temporal foraging of native ants and their richness on trees is strongly diminished when L. neglectus is present (Paris & Espadaler, 2012). Other arthropod groups also seem to be affected in positive (=enhanced presence; aphids), negative (=lesser density; lepidoptera larvae) or neutral ways.
Bertelsmeier et al. (2015a, b) examined elements of interspecific aggression, and food resource discovery and dominance, between this species and several other highly invasive ants. In laboratory assays Lasius neglectus was highly aggressive when confronted with workers of other invasive ants. Of the group of four species that were found to be aggressive, L. neglectus was found to be fairly adept at finding and recruiting to food in a laboratory arena experiment.
Effects at home
Not all populations seem to be invasive. In some of these, ants do not invade buildings or houses, and opt to nest outside, in public gardens, at the base of trees or in the cracks in cemented areas or sidewalks.
In certain populations (Seva, Taradell, and Matadepera in Spain, or Paris, in France) ants enter buildings and occupy diverse components of the construction. Killing ants by insecticide spray may produce impressive results. They seem to be attracted to electrical fields, causing failure and damage by shorting or by occupying electrical plugs, connexion boxes or electro-mechanical devices, such as automatic blinds.
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a host for the fungus Laboulbenia formicarum (a parasite) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission within nest).
- This species is a host for the fungus Laboulbenia formicarum (a pathogen) (Espadaler & Santamaria, 2012).
Tragust et al. (2015) - Two studied supercolonies of the invasive garden ant L. neglectus, surveyed over multiple years, showed the prevalence of the ectoparasitic fungus L. formicarum increased ca. 14% yearly from low levels until about 80 % of all worker ants were infected. In one of the supercolonies (Gif-sur-Yvette), all but one worker was infected and carried on average >200 thalli.
Invasiveness
Seifert (2020) - This invasive species has been the subject of perhaps a hundred publications during the last three decades. Invasion of Europe and Middle Asia started in about 1973. There is a clear potential for its spread into southern Scandinavia because some Asian populations survive in regions with mean January temperatures of –5°C.
Castes
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- neglectus. Lasius (Lasius) neglectus Van Loon, Boomsma & Andrasfalvy, 1990: 350, fig. 5 (w.q.m.) HUNGARY. Junior synonym of turcicus: Seifert, 1992b: 10. Revived from synonymy: Seifert, 1996b: 190 (in key); Seifert, 2000b: 176.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Taxonomic Notes
Lasius neglectus was described in 1990 (Van Loon et al. 1990) for specimens collected from a nest in the garden of the Company for the Development of Fruit and Ornamental Production at Budapest. The population there had been known since the early seventies (Andrásfalvy, in litt.). Lasius neglectus was shown to be allozymatically distinct (Boomsma et al. 1990) and was morphological compared with Lasius alienus and Lasius brunneus. The specific status of this ant was questioned shortly after its description (Seifert 1992) but is now undisputed.
Description
Worker
Seifert (2020) - Body size small (CS 772 µm). Number of mandibular dents low (MaDe900 7.3). Clypeal pubescence dilute (sqPDCL900 5.39). Pronotal setae rather short (PnHL/ CS900 0.127), not much longer than gular setae (GuHL/ CS900 0.115). Petiole scale in lateral view thin and forming an acute tip. Pubescence hairs on frons rather long (PLF 34.1 µm). Dorsum of scape and hind tibiae without or few, occasional setae. Coloration: Head, mesosoma and gaster dark brown; mandibles, antennae, tibiae and tarsae light yellowish-brown.
See table 2 in Seifert 2020 for additional morphometrics. The abbreviated names of various quantitative data shown above are defined here: Seifert 2020 Lasius characters.
Type Material
Seifert (2020) - 7 paratype workers from the holotype colony labelled ”HUNGARY Budapest 1. VII 1988“; depositories: The Natural History Museum London, Staatliches Museum für Naturkunde Görlitz.
References
- Arcos, J., Chaves, D., Alarcón, P., Rosado, A. 2022. First record of Temnothorax convexus (Forel, 1894) in Portugal (Hymenoptera: Formicidae) with an updated checklist of the ants from the country. Sociobiology, 69(2), e7623 (doi:10.13102/sociobiology.v69i2.7623).
- Arcos, J., Chaves, D., Alarcón, P., Rosado, Á. 2022. First record of Temnothorax convexus (Forel, 1894) in Portugal (Hymenoptera: Formicidae) with an updated checklist of the ants from the country. Sociobiology, 692), e7623 (doi:10.13102/sociobiology.v69i2.7623).
- Arnan, X., Angulo, E., Boulay, R., Molowny-Horas, R., Cerdá, X., Retana, J. 2021. Introduced ant species occupy empty climatic niches in Europe. Scientific Reports 11, 3280 (doi:10.1038/s41598-021-82982-y).
- Báthori, F., Herczeg, G., Vilizzi, L., Jégh, T., Kakas, C., Petrovics, M., Csősz, S. 2024. A survey and risk screening of non-native ant species colonising greenhouses in Hungary. Biological Invasions (doi:10.1007/s10530-023-03227-9).
- Báthori, F., Rádai, Z., Tartally, A. 2017. The effect of Rickia wasmanniii (Ascomycota, Laboulbeniales) on the aggression and boldness of Myrmica scabrinodis (Hymenoptera, Formicidae). Journal of Hymenoptera Research. 58:41–52. (doi:10.3897/jhr.58.13253)
- Baty, J.W., Bulgarella, M., Dobelmann, J., Felden, A., Lester, P.J. 2020. Viruses and their effects in ants (Hymenoptera: Formicidae). Myrmecological News 30: 213-228 (doi:10.25849/MYRMECOL.NEWS_030:213).
- Bertelsmeier, C., A. Avril, O. Blight, A. Confais, L. Diez, H. Jourdan, J. Orivel, N. St Germes, and F. Courchamp. 2015a. Different behavioural strategies among seven highly invasive ant species. Biological Invasions. 17:2491-2503. doi:10.1007/s10530-015-0892-5
- Bertelsmeier, C., A. Avril, O. Blight, H. Jourdan, and F. Courchamp. 2015b. Discovery-dominance trade-off among widespread invasive ant species. Ecology and Evolution. 5:2673-2683. doi:10.1002/ece3.1542
- Berville, L., Renucci, M. & Provost, E. 2012. Mise en place de protocoles de contrôle de la fourmi d'Argentine (Linepithema humile) sur les îles de Port-Cros et de Porquerolles (Var; France). Sci. Rep. Port-Cros natl. Park 26: 91-108.
- Boase, C. 2014. Lasius neglectus (Hymenoptera: Formicidae) in the UK: status, impact and management. Proc. Eighth Int. Conf. Urban Pests. G. Müller, R. Pospischil, W.H. Robinson (eds). OOK-Press Kft.Veszprém, Hungary: 223-228.
- Boomsma, J. J.; Brouwer, A. H.; Van Loon, A. J. 1990. A new polygynous Lasius species (Hymenoptera: Formicidae) from central Europe. II. Allozymatic confirmation of species status and social structure. Insectes Soc. 37: 363-375.
- Borowiec, L. 2014. Catalogue of ants of Europe, the Mediterranean Basin and adjacent regions (Hymenoptera: Formicidae). Genus (Wroclaw) 25(1-2): 1-340.
- Borowiec, L., Lebas, C., Salata, S. 2022. Notes on ants (Hymenoptera: Formicidae) from three northern Aegean islands – Lemnos, Samothraki and Thasos. Annals of the Upper Silesian Museum in Bytom, Entomology 31: 1-14 (doi:10.5281/ZENODO.7346453).
- Borowiec, L., Salata, S. 2018. Tetramorium immigrans Santschi, 1927 (Hymenoptera: Formicidae) nowy gatunek potencjalnie inwazyjnej mrówki w polsce. Acta entomologica silesiana 26:1-5 (doi:10.5281/ZENODO.1169156).
- Borowiec, L., van Delft, J.P.L., van Delft, J.J.C.W., Salata, S. 2023. Five ant species (Hymenoptera: Formicidae) new to the Greek fauna with notes on ants from Greek Thrace. Annales of the Upper Silesian Museum in Bytom, Entomology 32 (online 008), 1-13 (doi:10.5281/ZENODO.10101028).
- Borowiec, L., Wieczorek, K., Salata, S. 2021. Review of ants (Hymenoptera: Formicidae) of the Dodecanese Archipelago, Greece. Annals of the Upper Silesian Museum in Bytom Entomology 30: 1-33 (doi:10.5281/ZENODO.5571270).
- Bračko, G. 2019. New data on the ant fauna (Hymenoptera: Formicidae) of Azerbaijan. Caucasian Entomological Bulletin 15, 165–175 (doi:10.23885/181433262019151-165175).
- Castracani, C., Spotti, F.A., Schifani, E., Giannetti, D., Ghizzoni, M., Grasso, D.A., Mori, A. 2020. Public engagement provides first insights on Po Plain ant communities and reveals the ubiquity of the cryptic species Tetramorium immigrans (Hymenoptera, Formicidae). Insects 11, 678. (doi:10.3390/insects11100678).
- Castro Gil, A., Martínez de Murguía, L. & M.D. Martínez Ibáñez, 2010. Llegada de la hormiga exótica invasora Lasius neglectus Van Loon, Boomsma & Andrásfalvy, 1990 (Hymenoptera: Formicidae) a la Cornisa Cantábrica (España). Boletín de la Sociedad entomológica Aragonesa 47: 435-436.
- Cordonnier, M., Blight, O., Angulo, E., Courchamp, F. 2020. Behavioral data and analyses of competitive interactions between invasive and native ant species. Animals 10, 2451 (doi:10.3390/ani10122451).
- Cremer S, Ugelvig LV, Drijfhout FP, Schlick-Steiner BC, Steiner FM, Steiner, F.M., Seifert, B., Hughes, D.P., Schulz, A., Petersen, K.S., Konrad, H., Stauffer, C., Kiran, K., Espadaler, X., d’Ettorre, P., Aktaç, N., Eilenberg, J., Jones, G., Nash, D., Pedersen, J.S., Boomsma, J.J. 2008. The Evolution of Invasiveness in Garden Ants. PLoS ONE 3(12): e3838. doi:10.1371/journal.pone.0003838
- Cremer, S., Ugelvig, L.V., Drijfhout, F.P., Schlick-Steiner, B.C., Steiner, F.M., Seifert, B., Hughes, D.P., Schulz, A., Petersen, K.S., Konrad, H., Stauffer, C., Kiran, K., Espadaler, X., d'Ettorre, P., Aktaç, N., Eilenberg, J., Jones, G.R., Nash, D.R., Pedersen, J.S., Boomsma, J.J. 2008. The evolution of invasiveness in garden ants. PLoS ONE 3, e3838 (doi:10.1371/journal.pone.0003838).
- Cremer, S., Ugelvig, L.V., Lommen, S.T.E., Petersen, K.S. & Pedersen, J.S. 2006. Attack of the invasive garden ant: aggression behaviour of Lasius neglectus (Hymenoptera: Formicidae) against native Lasius species in Spain. Myrmecologische Nachrichten 9:13-19.
- Csata, E., Dussutour, A. 2019. Nutrient regulation in ants (Hymenoptera: Formicidae): a review. Myrmecological News 29: 111-124 (doi:10.25849/MYRMECOL.NEWS_029:111).
- Csősz, S., Báthori, F., Gallé, L., Lőrinczi, G., Maák, I., Tartally, A., Kovács, É., Somogyi, A.Á., Markó, B. 2021. The myrmecofauna (Hymenoptera: Formicidae) of Hungary: Survey of ant species with an annotated synonymic inventory. Insects 16;12(1):78 (doi:10.3390/insects12010078).
- Csosz, S., Marko, B., Galle, L. 2011. The myrmecofauna (Hymenoptera: Formicidae) of Hungary: an updated checklist. North-Western Journal of Zoology 7: 55-62.
- Czechowska, W. & Czechowski, W. 2003. Further record of Lasius neglectus Van Loon, Boomsma & Andrásfalvy (Hymenoptera: Formicidae) from Warsaw, with a key to the Polish species of the subgenus Lasius s.str. Fragm. faun. 46: 195-202.
- Czechowska, W. & W. Czechowski, 1999. Lasius neglectus Van Loon, Boomsma & Andrásfalvy, 1990 (Hymenoptera, Formicidae), nowy dla Polski gatunek mróvki w Warszawie. Przegląd Zoologiczny 43: 189-191.
- Czechowski, W., Radchenko, A., Czechowska, W. 2002. The ants (Hymenoptera, Formicidae) of Poland. MIZ PAS Warsaw.
- Dekoninck W., K. Lock and F. Janssens, 2007. Acceptance of two native myrmecophilous species, Platyarthrus hoffmannseggii and Cyphoderus albinus by the introduced invasive garden ant Lasius neglectus in Belgium. Eur. J. Entomol. 104: 159-161.
- Dekoninck, W., C. De Baere, J. Mertens & J-P. Maelfait, 2002. On the arrival of the Asian invader ant Lasius neglectus in Belgium (Hymenoptera, Formicidae). Bull. Soc. roy. belg. Ent. 138: 45-48.
- Dekoninck, W., Ignace, D., Vankerkhoven, F., Wegnez, P. 2012. Verspreidingsatlas van de mieren van België. Bulletin de la Société royale belge d’Entomologie 148: 95-186.
- Dekoninck, W., Wauters, N., Delsinne, T. 2019. Capitulo 35. Hormigas invasoras en Colombia. Hormigas de Colombia.
- Espadaler, X. & J.M. Olmo. 2011. The myrmecophilic cricket Myrmecophilus in Spain (Orthoptera, Myrmecophilidae). Sociobiology 57: 321-328.
- Espadaler, X. & S. Rey, 2001. Biological constraints and colony founding in the polygynous invasive ant Lasius neglectus (Hymenoptera, Formicidae). Insectes soc. 48: 159-164.
- Espadaler, X. & V. Bernal, 2003. Exotic ants in the Canary Islands, Spain (Hymenoptera, Formicidae). Vieraea 31: 1-7.
- Espadaler, X. 1999. Lasius neglectus Van Loon, Boomsma & Andrásfalvy, 1990 (Hymenoptera, Formicidae), a potential pest ant in Spain. Orsis 14: 43-46.
- Espadaler, X., Lebas, C., Wagenknecht, J. & S. Tragust. 2011. Laboulbenia formicarum (Ascomycota, Laboulbeniales), an exotic parasitic fungus, on an exotic ant in France. Vie et Milieu 61: 41-44.
- Espadaler, X., Rey, S. & V. Bernal, 2004. Queen number in a supercolony of the invasive garden ant, Lasius neglectus. Insectes soc. 51: 232-238.
- Espadaler, X., Santamaria, S. 2012. Ecto- and Endoparasitic Fungi on Ants from the Holarctic Region. Psyche Article ID 168478, 10 pages (doi:10.1155/2012/168478).
- Espadaler, X., Tartally, A., Schultz, R., Seifert, B., Nagy, C. 2007. Regional trends and preliminary results on the local expansion rate in the garden invasive ant, Lasius neglectus (Hymenoptera, Formicidae). Insectes Sociaux 54 : 293-301.
- Fournier, D., Tindo, M., Kenne, M., Mbenoun Masse, P.S., Van Bossche, V., De Coninck, E., Aron, S. 2012. Genetic structure, nestmate recognition and behaviour of two cryptic species of the invasive Big-Headed Ant Pheidole megacephala. PLoS ONE 7(2): e31480 (doi:10.1371/journal.pone.0031480).
- Fox, M. 2010. First incursion of Lasius neglectus (Hymenoptera: Formicidae), an invasive polygynous ant in Britain. British Journal of Entomology and Natural History 23: 1803: 1-3.
- Frizzi, F., F. Talone, and G. Santini. 2018. Modulation of trail laying in the ant Lasius neglectus (Hymenoptera: Formicidae) and its role in the collective selection of a food source. Ethology. 124:870-880. doi:10.1111/eth.12821
- Frizzi, F., Masoni, A., Migliorini, M., Fanciulli, P.P., Cianferoni, F., Balzani, P., Giannotti, S., Davini, G., Frasconi Wendt, C., Santini, G. 2020. A comparative study of the fauna associated with nest mounds of native and introduced populations of the red wood ant Formica paralugubris. European Journal of Soil Biology 101, 103241. (doi:10.1016/j.ejsobi.2020.103241).
- Gippet, J.M.W., Colin, T., Grangier, J., Winkler, F., Haond, M., Dumet, A., Tragust, S., Mondy, N., Kaufmann, B. 2021. Land-cover and climate factors contribute to the prevalence of the ectoparasitic fungus Laboulbenia formicarum in its invasive ant host Lasius neglectus. Fungal Ecology 51, 101045 (doi:10.1016/j.funeco.2021.101045).
- Herraiz J.A. & X. Espadaler, 2007. Laboulbenia formicarum (Ascomycota, Laboulbeniales) reaches the Mediterranean. Sociobiology 50: 449-455.
- Herraiz, J.A., Espadaler, X. 2012. Estudi de les comunitats de formigues del mosaic de bosc de ribera del Parc Natural de Sant Llorenç del Munt i l’Obac. VII Trobada d’estudiosos de Sant Llorenç del Munt i l’Obac:53-61. Diputació de Barcelona
- Jucker, C., F. Rigato & R. Regalin. 2008. Exotic ant records from Italy (Hymenoptera, Formicidae). Boll. Zool. agr. Bachic. Ser. II, 40: 99-107.
- Khalini-Moghadam, A., Borowiec, L., Nemati, A. 2019. New records of ants (Hymenoptera: Formicidae) from the Chaharmahal va Bakhtiari Province of Iran with taxonomic comments. Polish Journal of Entomology 88: 163–182 (DOI 10.2478/pjen-2019-0013).
- Kiran, K., Karaman, C. 2020. Additions to the ant fauna of Turkey (Hymenoptera, Formicidae). Zoosystema 42(18), 285-329 (doi:10.5252/zoosystema2020v42a18).
- Konrad, M., Grasse, A.V., Tragust S., Cremer, S. 2015. Anti-pathogen protection versus survival costs mediated by an ectosymbiont in an ant host. Proceedings of the Royal Society, B 282(1799). doi: 10.1098/rspb.2014.1976.
- Lapeva-Gjonova, A., Antonova, V. 2022. An updated checklist of ants (Hymenoptera, Formicidae) of Bulgaria, after 130 years of research. Biodiversity Data Journal 10, e95599 (doi:10.3897/bdj.10.e95599).
- Loon, A.J. van 2012. De plaagmier Lasius neglectus in Nederland. Forum Formicidarum 10 (1-3) [2009]: 2-6.
- Markó, B. 1998. Six new ant species (Hymenoptera: Formicidae) for the Romanian myrmecofauna. Entomol. rom. 3: 119-123.
- Marlier, J.F., B. Schatz & J.C. de Biseau, 2002. Influence de Crematogaster scutellaris (Hymenoptera: Myrmicinae) sur deux communautés de fourmis. Colloque UIEIS, Versailles: 68-72.
- Martelloni, G., Santarlasci, A., Franco Bagnoli, F., Santini, G. 2015. Modeling ant battles by means of a diffusion-limited Gillespie algorithm. Theoretical Biology Forum 107: 57-76.
- Meurville, M.-P., LeBoeuf, A.C. 2021. Trophallaxis: the functions and evolution of social fluid exchange in ant colonies (Hymenoptera: Formicidae). Myrmecological News 31: 1-30 (doi:10.25849/MYRMECOL.NEWS_031:001).
- Molfini, M., Zapparoli, M., Genovesi, P., Carnevali, L., Audisio, P., Di Giulio, A., Bologna, M.A. 2020. A preliminary prioritized list of Italian alien terrestrial invertebrate species. Biological Invasions 22(8), 2385–2399 (doi:10.1007/s10530-020-02274-w).
- Nagy, C., Tartally, A., Vilisics, F., Merkl, O., Szita, É., Szél, G., Podlussány, A., Rédei, D., Csösz, S., Pozsgai, G., Szövény, G., Markó, V. 2009. Effects of the invasive garden ant, Lasius neglectus Van Loon, Boomsma et Andrásfalvy, 1990 (Hymenoptera, Formicidae) on arthropod assemblages: pattern analyses in the type supercolony. Myrmecological News 12: 171-181. (supplementary material )
- Neumeyer, R. 2008. Ergänzungen zur Artenliste der frei lebenden Ameisen (Hymenoptera: Formicidae) in der Schweiz. Entomo Helvetica 1: 43-48.
- Paris C. I., Llusiá J. & J. Peñuelas. 2010. Changes in monoterpene emission rates of Quercus ilex infested by aphids tended by native or invasive Lasius ant species. Journal of Chemical Ecology 36:689–698.
- Paris, C. & Espadaler, X. 2012. Foraging activity of native ants on trees in forest fragments colonized by the invasive ant Lasius neglectus. Psyche 2012 (261316): 1-9.
- Paris, C. 2005. Mutualismo de la hormiga invasora Lasius neglectus (Hymenoptera: Formicidae) y el áfido Lachnus roboris (Homoptera: Lachnidae) en un encinar urbano. Ms Thesis UAB (in spanish).
- Paris, C.I. & X. Espadaler. 2009. Honeydew collection by the invasive garden ant Lasius neglectus versus the native ant L. grandis. Arthropod-Plant Interactions 3: 75-85.
- Pashaei Rad, S., Taylor, B., Torabi, R., Aram, E., Abolfathi, G., Afshari, R., Borjali, F., Ghatei, M., Hediary, F., Jazini, F., Heidary Kiah, V., Mahmoudi, Z., Safariyan, F., Seiri, M. 2018. Further records of ants (Hymenoptera: Formicidae) from Iran. Zoology in the Middle East 64, 145-159 (doi:10.1080/09397140.2018.1442301).
- Passera, L. 1994. Characteristics of tramp species. In: Williams, D.F. (ed.). Exotic ants. Biology, impact, and control of introduced species. Westview Press: p. 23-43.
- Pawluk, F., Borowiec, L., Salata, S. 2022. First record of Plagiolepis alluaudi Emery, 1894 (Hymenoptera: Formicidae) from Poland. Annals of the Upper Silesian Museum in Bytom Entomology 31 (online 006) 1-5 (doi:10.5281/ZENODO.6522444).
- Rey, S. & X. Espadaler. 2005. Area-wide management of the invasive garden ant Lasius neglectus (Hymenoptera: Formicidae) in Northeast Spain. J. Agric. Urban Entomol. 21: 99-112.
- Schultz, R. & B. Seifert. 2005. Lasius neglectus (Hymenoptera: Formicidae) -a widely distributed tramp species in Central Asia. Myrmecologische Nachrichten 7: 47-50.
- Schultz, R. & T. Busch. 2009: The northernmost record of the invasive garden ant, Lasius neglectus (Hymenoptera: Formicidae). Myrmecological News 12: 183-186.
- Seifert, B. 1992. A taxonomic revision of the Palaearctic members of the ant subgenus Lasius s.str. (Hymenoptera: Formicidae). Abh. Ber. Naturkundemus. Görlitz 66: 1-67.
- Seifert, B. 2000. Rapid range expansion in Lasius neglectus (Hymenoptera, Formicidae)- an Asian invader swamps Europe. Mitt. Mus. Nat. kd. Berl., Dtsch. Entomol. Z. 47: 173-179.
- Seifert, B. 2020. A taxonomic revision of the Palaearctic members of the subgenus Lasius s.str. (Hymenoptera, Formicidae). Soil Organisms 92(1): 15-86 (doi:10.25674/so92iss1pp15)
- Shang, Y., Feng, P., Wang, C. 2015. Fungi that infect insects: Altering host behavior and beyond. PLOS Pathogens 11, e1005037 (doi:10.1371/journal.ppat.1005037).
- Siddiqui, J. A., Li, J., Zou, X., Bodlah, I., Huang, X. 2019. Meta-analysis of the global diversity and spatial patterns of aphid-ant mutualistic relationships. Applied Ecology and Environmental Research 17: 5471-5524 (doi:10.15666/aeer/1703_54715524).
- Siddiqui, J.A., Bamisile, B.S., Khan, M.M., Islam, W., Hafeez, M., Bodlah, I., Xu, Y. 2021. Impact of invasive ant species on native fauna across similar habitats under global environmental changes. Environmental Science and Pollution Research 28(39), 54362–54382 (doi:10.1007/s11356-021-15961-5).
- Steiner, F.M., B.C. Schlick-Steiner, S. Schödl, X. Espadaler, B. Seifert, E. Christian & C. Stauffer. 2004. Phylogeny and bionomics of Lasius austriacus (Hymenoptera, Formicidae). Insectes soc. 51: 24-29.
- Stukalyuk, S., Radchenko, A., Akhmedov, A., Reshetov, A., Netsvetov, M. 2021. Acquisition of invasive traits in ant, Crematogaster subdentata Mayr (Hymenoptera: Formicidae) in urban environments. Serangga 26: 1-29.
- Stukalyuk, S.V., Radchenko, A., Reshetov, A., Akhmedov, A., Goncharenko, I. 2021. Comparative analysis of the population structure of Crematogaster subdentata and Lasius neglectus in the primary and secondary ranges (Hymenoptera: Formicidae). Fragmenta Entomologica 53, 43-51 (doi:10.13133/2284-4880/436).
- Stukalyuk, S.V., Radchenko, A.G., Akhmedov, A., Reshetov, A.A. 2020. Uzbekistan - The alleged native range of the invasive ant Lasius neglectus (Hymenoptera, Formicidae): Geographical, ecological and biological evidences. Zoodiversity 54(2): 111-122 (doi:10.15407zoo2020.02.111).
- Tartally, A. & F. Báthori. 2015. Does Laboulbenia formicarum (Ascomycota: Laboulbeniales) fungus infect the invasive garden ant, Lasius neglectus (Hymenoptera: Formicidae), in Hungary? e-Acta Naturalia Pannonica 8: 117–123.
- Tartally, A. 2000. A Magyarországról leírt invázív Lasius neglectus van Loon, Boomsma et Andrásfalvy, 1990 (Hymenoptera: Formicidae) újabb hazai lelöhelyei. Fol ent. hung. 61: 298-300.
- Tartally, A. 2000. Notes on the coexistence of the supercolonial Lasius neglectus Van Loon, Boomsma et Andrásfalvy 1990 (Hymenoptera: Formicidae) with other ant species. Tiscia 32: 43-46.
- Tartally, A. 2006. Long term expansion of a supercolony of the invasive garden ant Lasius neglectus (Hymenoptera: Formicidae). Myrmecologische Nachrichten 9: 21-25. .
- Tartally, A., E. Hornung & X. Espadaler, 2004. The joint introduction of Platyarthrus schoblii (Isopoda: Oniscidea) and Lasius neglectus (Hymenoptera: Formicidae) into Hungary. Myrmecologische Nachrichten 6: 61-66.
- Tragust, S., H. Feldhaar, X. Espadaler, and J. S. Pedersen. 2015. Rapid increase of the parasitic fungus Laboulbenia formicarum in supercolonies of the invasive garden ant Lasius neglectus. Biological Invasions. 17:2795-2801. doi:10.1007/s10530-015-0917-0
- Trigos-Peral, G., Abril, S., Angulo, E. 2020. Behavioral responses to numerical differences when two invasive ants meet: the case of Lasius neglectus and Linepithema humile. Biological Invasions (doi:10.1007/s10530-020-02412-4).
- Ugelvig, L.V. & S. Cremer. 2007. Social prophylaxis: group interaction promotes collective immunity in ant colonies. Current Biology, 17(22): 1972-1977.
- Ugelvig, L.V.; Drijfhout, F.P.; Kronauer, D.J.C.; Boomsma, J.J.; Pedersen, J.S. & S. Cremer. 2008. The introduction history of invasive garden ants in Europe: integrating genetic, chemical and behavioural approaches. BMC Biology 6: 11.
- Van Loon, A. J.; Boomsma, J. J.; Andrasfalvy, A. 1990. A new polygynous Lasius species (Hymenoptera: Formicidae) from central Europe. I. Description and general biology. Insectes Soc. 37: 348-362.
- Артохин, К.С., Игнатова, П.К., Колесников, С.И., Решетов, А.A. 2013. Изменения в фауне перепончатокрылых насекомых Ростовской области ипрогноз экологических последствий. Электронное периодическое издание ЮФУ «Живые и биокосные системы», №2, 2013. (Artohin K.S., Ignatova, P.K., Kolesnikov, S.I. & Reshetov, A.A. 2013. Changes in fauna of Hymenoptera insects of the Rostov area and forecast of environmental effects. Living and bio-inert systems 2: 1-10.)
References based on Global Ant Biodiversity Informatics
- Borowiec L. 2014. Catalogue of ants of Europe, the Mediterranean Basin and adjacent regions (Hymenoptera: Formicidae). Genus (Wroclaw) 25(1-2): 1-340.
- Borowiec L., and S. Salata. 2012. Ants of Greece - Checklist, comments and new faunistic data (Hymenoptera: Formicidae). Genus 23(4): 461-563.
- Dubovikoff D. A., and Z. M. Yusupov. 2018. Family Formicidae - Ants. In Belokobylskij S. A. and A. S. Lelej: Annotated catalogue of the Hymenoptera of Russia. Proceedingss of the Zoological Institute of the Russian Academy of Sciences 6: 197-210.
- Ghahari H., C. A. Collingwood, M. Tabari, and H. Ostovan. 2009. Faunistic notes on Formicidae (Insecta: Hymenoptera) of rice fields and surrounding grasslands in northern Iran. Mun. Ent. Zool. 4(1): 184-189.
- Khalili-Moghadam A., L. Borowiec, and A. Nemati. 2019. New records of ants (Hymenoptera: Formicidae) from the Chaharmahal va Bakhtiari Province of Iran with taxonomic comments. Polish Journal of Entomology 88 (2): 163–182.
- Omid P., and H. G. Kami. 2007. New and additional records for the formicid fauna (Insecta: Hymenoptera) of Iran. Zoology in the Middle East 40(1): 85-90.
- Paknia O., A. Radchenko, H. Alipanah, and M. Pfeiffer. 2008. A preliminary checklist of the ants (Hymenoptera: Formicidae) of Iran. Myrmecological News 11: 151-159.
- Pashaei Rad S., B. Taylor, R. Torabi, E. Aram, G. Abolfathi, R. Afshari, F. Borjali, M. Ghatei, F. Hediary, F. Jazini, V. Heidary Kiah, Z. Mahmoudi, F. Safariyan, and M. Seiri. 2018. Further records of ants (Hymenoptera: Formicidae) from Iran. Zoology in the Middle East 64(2): 145-159.


