Pristomyrmex punctatus
| Pristomyrmex punctatus | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Crematogastrini |
| Genus: | Pristomyrmex |
| Species: | P. punctatus |
| Binomial name | |
| Pristomyrmex punctatus (Smith, F., 1860) | |
| Synonyms | |
| |
| Common Name | |
|---|---|
| Amime-ari | |
| Language: | Japanese |
| Notes: | as Pristomyrmex pungens |
Wang (2003) - It appears that P. punctatus occurs in open habitats. My impression stems from field experience in China as well as from data associated with specimens. This species lacks a normal queen caste; mature colonies usually contain several thousand workers and a few males, but ergatoid queens are rarely found. Eggs are normally laid by workers and can develop into workers or males. Ergatoid queens, when present, can also lay eggs. Brood production begins in April and lasts until the end of September. Young workers remain inside the nest and lay eggs. Older workers forage but lose the ability to lay eggs. Nests are often constructed in leaf litter from June to August but in the soil around trees from September to October. Sometimes arboreal nests are constructed on dead standing trees or in partially dead parts of living trees. The nest entrances of the arboreal nests and those under rotten wood are often covered with soil particles. See the following references for information on these and other aspects of the biology of P. punctatus (How et al., 1984; Mizutani, 1980, 1982; Tsuji, 1988a, 1988b, 1988c, 1990a, 1990b, 1994, 1995; Tsuji and Ito, 1986).
| At a Glance | • Parthenogenetic • Polygynous |
Photo Gallery
 Pristomyrmex punctatus workers collecting honeydew from aphids in Japan. Photo by Seidai Nagashima.
Pristomyrmex punctatus workers collecting honeydew from aphids in Japan. Photo by Seidai Nagashima. Pristomyrmex punctatus workers gathering honeydew from scale insects in Japan. Photo by Seidai Nagashima.
Pristomyrmex punctatus workers gathering honeydew from scale insects in Japan. Photo by Seidai Nagashima.
Identification
Wang (2003) - Eyes with eight or more ommatidia in the longest row; pronotum unarmed; propodeal spines straight, and long, much longer than the distance between their bases; dorsal surfaces of both head and alitrunk covered fully with rugoreticulum; dorsum of petiole node with two or more pairs of hairs.
The separation of P. punctatus from both Pristomyrmex divisus and Pristomyrmex pulcher is summarized in the discussions of both P. divisus and P. pulcher. The following characters can be used to separate the workers of P. punctatus from those of its closest relative, Pristomyrmex rigidus: P. punctatus - Dorsum of petiole node with two or more pairs of hairs. Tooth absent or inconspicuous from basal margin of mandible. Dorsal surfaces of head and alitrunk, and the sides of petiole and postpetiole more finely sculptured. Propodeal spines relatively slender. Clypeus with a median longitudinal carina that meets the anterior clypeal margin. Dorsum of alitrunk in dorsal view, marginated, and more or less depressed. Ventral surface of clypeus with a curved ruga but lacking distinct toothlike prominences. P. rigidus - Dorsum of petiole node only with a single pair of hairs. In type specimens, a strongly prominent tooth present on basal margin of mandible. Dorsal surfaces of head and alitrunk, and the sides of petiole and postpetiole more coarsly sculptured. Propodeal spines relatively robust. Median clypeal carina often not reaching the anterior clypeal margin. Dorsum of alitrunk in dorsal view, convex. Ventral surface of clypeus usually with two minute toothlike prominences.
In addition, an ergatoid queen caste is present in P. punctatus but not seen in P. rigidus, while a normal queen caste exists in P. rigidus but has not been found in P. punctatus.
A member of the Punctatus species group
Keys including this Species
- Key to Micronesian Ants
- Key to Pristomyrmex of China
- Key to Pristomyrmex of the Philippines
- Key to Pristomyrmex workers
Distribution
Latitudinal Distribution Pattern
Latitudinal Range: 38.734° to -1.5°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Indo-Australian Region: Borneo, Indonesia (type locality), Malaysia, New Guinea, Philippines, Singapore.
Oriental Region: Laos, Sri Lanka, Taiwan, Thailand, Vietnam.
Palaearctic Region: China, Democratic Peoples Republic of Korea, Japan, Republic of Korea.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Biology
Wang (2003) - P. punctatus originated in the subtropics or tropics of Asia, as its three close relatives, P. rigidus, P. pulcher, and P. divisus, are restricted to Sumatra, Malaysia, Brunei, Thailand, and the Philippines, respectively. But P. punctatus has a very large range, from New Guinea, Indonesia to Malaysia, Thailand, and then north to China and Japan, indicating its exceptional dispersal ability and tolerance of temperate climates. Pristomyrmex punctatus has shown some tendency for dispersal by humans. Interception records from ports in North America suggest that human commerce may have played a role in this species' spread in temperate Asia.
The chromosome numbers of this species, reported by Imai (1966) and Itow et al. (1984), are 211 (diploid) = 24 in the cerebral ganglion cells of the workers and 11 (haploid) = 12 in the spermatocytes of the males. The larva of this species was described by Wheeler and Wheeler (1954).
I have also examined 11 workers collected at the two entry ports of the United States (USNM): nine of them, by Harley and Albrecht, on November 20, 1928, from Philadelphia, Pennsylvania, in lily bulbs imported from Japan; the other two specimens, by J. F. Byrnes, on September 25, 1967, from Anchorage, Alaska, on Gerberia sp. imported from Japan. It appears, however, that P. punctatus has not yet become established in the United States (Cover, personal communication).
Yamada and Eguchi (2016) reported a colony of P. punctatus was found under a concrete block on the ground in a green tract of the Minami-Osawa Campus of Tokyo Metropolitan University. The colony contained more than 100 males. Some of these individuals subsequently served as the source material for their description of the male genitalia of this species. The finding and description of these males are important, in part, as this species can reproduce parthenogenetically. Despite this, the male genitalia does appear to be fully functional in this species when males are produced.
Japan
This species is queenless. The workers produce further workers by thelytoky (Mizutani, 1980, 1984; Itow et al., 1984). Functionally, two classes of workers are present in colonies: extranidal workers, which forage outside the nest, and intranidal workers, which stay within. The intranidal workers are the reproductives and lay eggs. Younger workers are intranidal, older ones extranidal (Mizutani, 1980, 1984; Tsuji, 1988, 1988a, 1990a). In some cases colonies may have many ergatoid females. These have relatively large heads and ocelli (Itow et al., 1984). Males are rarely produced, if so they are found in summer. A large colony of this species can comprise several tens of thousands to several hundred thousand workers. P. pungens does not establish permanent nests, but relocates its colonies frequently (Mizutani, 1982, 1984; Tsuji, 1988b). Long lines of migrating ants are often formed. Ecological and ethological studies have been carried out by Morisita (1939), Tsuji & Ito (1986) and Tsuji (1990b). Forel (1900) described Pristomyrmex japonicus from Japanese material, but Viehmeyer (1922) later synonymized japonicus with P. pungens. The species is widely distributed in Southeast Asia. P. pungens is commonly found in Japan, but is less common and sometimes rare in other areas. (Japanese Ant Image Database)
Myrmechochory
Zhu and Wang (2014) found this species was an important disperser of "Corydalis wilfordii and C. racemosa (Papaveraceae) seeds in a subtropical evergreen forest at the Jiugongshan mountain (Hubei Province, China). The seed size of C. wilfordii is larger than that of C. racemosa, while the elaiosome/seed mass ratio of C. wilfordii is greater than that of C. racemosa. Being the mutual ant dispersers of the two Corydalis taxa, Pristomyrmex pungens (=Pristomyrmex punctatus) (with mass recruitment mode, i.e. to recruit a large number of ants to remove seeds when one ant found seed) and Prenolepis sphingthorax (=Nylanderia flaviabdominis) (with simple cooperative recruitmen mode, i.e. to recruit 5-30 ants to remove seeds when one ant found seed) played a varied role in seed dispersal of two plants. Pristomyrmex pungens carried about 44% of the C. wilfordii seeds transported by it to the nests, with the average dispersal distance of 1.85 m and the removal number of seeds per hour of 43.8, while the rest seeds were removed elaiosomes in situ and/or on the way to the nest. Both Pristomyrmex pungens and Prenolepis sphingthorax carried the all seeds of C. racemosa to the nests, with the average dispersal distance of 6.27 m and 6.65 m, and removal number of seeds per hour of 34.2 and 10.6 respectively. The results suggested that seed removal rate of ant with mass recruitment was higher than that of ant with simple cooperative recruitment. The seed dispersal distance and short-term seed fate differed between the two studied plants, depending on ant foraging strategies and behavior and seed characteristics."
Zhu and Wang (2018) reported on the responsiveness of myrmecochorous ants to leaf volatiles of Corydalis and the potential role of these chemicals in attracting ants to a plant's seeds. "Although the myrmecochorous seeds can be found and then transported by ants because of the attached elaiosome, the results of our study indicated that leaf volatiles of some myrmecochorous Corydalis species can function as an attractant for ant dispersers, and such attractiveness in turn enhances the seed retrieval. It suggests that leaf volatiles of some Corydalis species may be one of several factors shaping the ant-seed dispersing interactions. Further research is needed to identify the chemical basis of volatiles that trigger ant foraging activity, and to assess to what extent the seed dispersal of Corydalis species depend on leaf volatiles."
Morphology
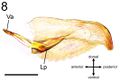
Male Genitalia - Yamada and Eguchi (2016) - Pygostyle digitiform with 3 – 8 long setae in its apical third (Fig. 2). Abdominal sternite IX subhexagonal in outline (Fig. 3); spiculum (anterior apophysis of the sternite IX, Sp in Fig. 3) short; anterior margins meeting in an obtuse angle basal to the spiculum; anterolateral sternal corners weakly produced anterolaterally; lateral margins slightly concave; posterolateral sternal corners somewhat produced anterolaterally; posteromedian part ventrally with long setae. Genital capsule longer than wide. Cupula wider than long, weakly constricted anteriorly (Fig. 4), with its mesoventral part shortened anteroposteriorly as a transverse bridge (the anterior and posterior margins of cupula indicated by black dashed line in Fig. 4). Telomere distinctly differentiated from basimere by ventral notch (the notch indicated by dashed line in Fig. 5); oblique carina present on lower of basimere (BmC in Fig. 5); gonostipital arm (median anteroventral extension of the basimere indicated by red dashed line in Fig. 4 and Ga in Fig. 4 and 5) in ventral view almost as long as basal width, with its lateral and mesal margins converging and forming an acute apex; telomere in lateral view almost as long as high, with setae on the outer surface of its posterior part (Fig. 6); ventral ridge of volsella with setae (Fig. 5); cuspis in lateral view roundly lobate and short, not reaching posterior margin of digitus (Fig. 7); digitus in lateral view claw-shaped, entirely hooked ventrad (Fig. 7); very short setae with distinct sockets scattered ventrally on digitus. Valviceps in lateral view with posterior apex hooked ventrad, anteroventral corner not produced; ventral margin with 9 – 13 denticles (Fig. 8); foveae (recognized as tunnels running inside valviceps) sparsely present on apical and ventral area of valviceps (some of apical foveae probably with short setae but obscure in optical microscope observation); valvura directed dorsoanterolaterally (Va in Fig. 9); lateral apodeme produced ventrolaterally (Lp in Fig. 9), forming wing-like structure which hold basal part of volsella in genital complex; penisvalva membrane densely spinate (Pvm in Fig. 9).
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a mutualist for the aphid Aphis craccivora (a trophobiont) (Katayama et al., 2013; Saddiqui et al., 2019).
- This species is a mutualist for the aphid Aphis gossypii (a trophobiont) (Kaneko, 2003; Saddiqui et al., 2019) (as Pristomyrmex pungens).
Castes
Worker
   
| |
| Worker. . | Owned by Museum of Comparative Zoology. |
Male

| |
| Worker. Photographer Yamada and Eguchi, 2016. | |
See also the Morphology section above.
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- punctatus. Myrmica punctata Smith, F. 1860b: 108 (w.) INDONESIA (Batjan I.). Wang, M. 2003: 412 (ergatoid q.). Combination in Pristomyrmex: Mayr, 1886c: 361. Senior synonym of pungens (and its junior synonym japonicus): Wang, M. 2003: 410.
- pungens. Pristomyrmex pungens Mayr, 1866b: 904, pl. 20, fig. 13 (w.) WEST MALAYSIA. Wheeler, W.M. 1928d: 114 (m.); Wheeler, G.C. & Wheeler, J. 1954c: 131 (l.); Imai, 1966: 119 (k.). Senior synonym of japonicus: Viehmeyer, 1922: 207. See also: Itow, Kobayashi, et al. 1984: 87; Lin & Wu, 1998: 94. Junior synonym of punctatus: Wang, M. 2003: 410.
- japonicus. Pristomyrmex japonicus Forel, 1900e: 268 (w.) JAPAN. Junior synonym of pungens: Viehmeyer, 1922: 207.
Type Material
The following notes on F. Smith type specimens have been provided by Barry Bolton (details):
Myrmica punctata
Two worker syntypes in Oxford University Museum of Natural History. Labelled “Bac 24.” (= Batjan I.).
Wang (2003):
Syntype workers, Indonesia: Bachian I. (A. H. Wallace) (Oxford University Museum of Natural History) [examined].
Pristomyrmex pungens Holotype worker, Malaysia: Malacca (?) (NHPS) [examined].
Pristomyrmex japonicus Syntype worke rs , Japan: Osaka (K. Yamada) (Musee d'Histoire Naturelle Genève) [examined]. Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Wang (2003) - TL 2.62-3.22, HL 0.70-0.84, HW 0.68-0.84, CI 94-105, SL 0.78-0.86, SI 102-118, EL 0.1.5-0.18, PW 0.48-0.56, AL 0.70-0.84, PPW 0.24-0.27, PPL 0.17-0.20, PPI 126-163 (11 = 70).
Mandibles usually with a few fine longitudinal rugae but smooth near the masticatory margin. Dentition of the masticatory margin of mandible: the strongest apical tooth + the second strongest pre apical + a long diastema + a broad basal tooth (or two small basal denticles). Basal margin of mandible rather straight, lacking a distinct tooth. Clypeus shieldlike, more or less depressed, with a median longitudinal carina extending posteriorly through the frontal area. In some specimens, a few short weak rugae present on each side of the median carina of the clypeus. Anterior clypeal margin equipped with a row of denticles, but sometimes median denticle indistinct or absent or becoming a broad-truncated lobe. Lateral portions of clypeus reduced to margins, so that the antennal fossae reach the anterior clypeal margin. Ventral surface of clypeus usually with a curved ruga. Palp formula 5,3. Frontal carinae distinct, extending to the level of the posterior margins of eyes. Antennal scrobes weak. Frontal lobes almost completely absent; thus, the antennal articulations are entirely exposed. Antennal scapes, when lying on the dorsal head, surpassing the occipital margin of head by one-sixth to one-fifth of their length. Eyes large, with eight or more ommatidia in the longest row. Occipital margin feebly concave. Dorsum of the alitrunk in dorsal view marginated and more or less depressed. Pronotum unarmed. Propodeal spines long, acute, but slender. Metapleural lobes dentiform and acute. Petiole node in profile wedge-shaped, with a triangular apex. Postpetiole in profile convex dorsally; in dorsal view transverse-rectangular, much broader than long, and also broader than the petiole. Dorsal surfaces of head and alitrunk as well as sides of pronotum cove red fully with rugoreticulum, but scrobal areas with only several transverse rugae. Sides of the rest of the alitrunk with numerous irregular rugae. Sides of petiole and postpetiole usually with a few fine longitudinal rugae; sometimes rugae very weak and broken. Gaster unsculptured. Dorsal surfaces of head and alitrunk with numerous erect to suberect long hairs. Two (or more) pairs of hairs present, respectively, bilaterally on the dorsums of petiole node and postpetiole, of which usually a pair shorter and the other pair longer. First gastral tergite lacking erect or suberect hairs. A few pairs of long, forward-projecting hairs present near the anterior clypeal margin that are symmetrical on the two sides of the midpoint. Scapes and tibiae with numerous erect to suberect short hairs. Color reddish-brown, but sometimes the gaster much darker or the appendages slightly lighter.
Queen
Wang (2003) – Ergatoid. TL 3.60, 3.72; HL 0.86, 0.88; HW 0.94, 0.94; CI 107, 109; SL 0.89, 0.91; SI 97, 97; EL 0.23, 0.23; PW 0.66, 0.68; AL 0.94, 0.96; PPW 0.31 , 0.32; PPL 0.23, 0.24; PPI 133, 135 (11 = 2).
Closely resembling the worker in the structure of mandibles, clypeus, petiole, postpetiole and gaster and also in sculpture, color, and pilosity. But the head with three ocelli; eyes large r; pronotum and propodeum narrower than those of worker; mesonotum more convex; an impression present at the approximate positions of promesonotal suture and of metanotal groove, respectively; propodeal spines stronger than in worker. Wing absent, but the rudimentary vestige of a wing is present on the each lateral margin of the mesonotum.
Male
Wang (2003) - TL 3.22, HL 0.60, HW 0.57, CI 9,5, SL 0.18, SI 32, HWE 0.79, EL 0.32, PW 0.74, AL 1.04, PPW 0.26, PPL 0.17, PPI 153 (11 = 1; one specimen [MCZC] collected from Nara, Japan, by Silvestri on July 21, 1925, was examined).
Head, including the eyes, broader than long. Clypeus transverse, with a median short carina. Frontal area with a median longitudinal carina. Frontal carinae short, slightly beyond the posterior margins of antennal sockets. Palp formula 5,3. On the mesoscutum, notauli pronounced, forming a Y shape; parapsidal furrows superficially impressed. Propodeum with a pair of teeth. Metapleural lobes subtriangular. Middle and hind tibiae each with a simple spur. Petiole node wedge-shaped, with a triangular apex; dorsum of petiole peduncle forming a declivity that reaches the top of the node. Postpetiole in profile rounded dorsally, in dorsal view transverse-rectangular and distinctly broader than long. Dorsum of head smooth and shining, except for few short rugae present behind the posterior margin of clypeus. Pronotum and mesoscutum smooth, except for those marked sutures, but mesoscutellum and propodeum sculptured with several longitudinal algae. Sides of petiole with a few rugae. All dorsal surfaces with abundant erect or suberect hairs, but hairs on the legs and on the scapes somewhat appressed. Colour reddish-brown; wing light-yellow.
Karyotype
- n = 12, 2n = 24 (Japan) (Imai & Yosida, 1964; Imai, 1966; Imai, 1969; Itow et al., 1984) (as Pristomyrmex pungens).
References
- Gotoh, A., Billen, J., Tsuji, K., Sasaki, T., Ito, F. 2011. Histological study of the spermatheca in three thelytokous parthenogenetic ant species, Pristomyrmex punctatus, Pyramica membranifera and Monomorium triviale (Hymenoptera: Formicidae). Acta Zool 93: 200–207.
- Harada, Y. 2011. Arboreal ant fauna of Joyama Park, Kagoshima Prefecture, southern Japan. Asian Myrmecology. 4:79-87. doi:10.20362/am.004005
- Hasegawa, E., Kobayashi, K., Yagi, N. & Tsuji, K. 2011. Complete mitochondrial genomes of normal and cheater morphs in the parthenogenetic ant Pristomyrmex punctatus (Hymenoptera: Formicidae). Myrmecological News, 15, 85-90.
- Hashimoto, Y. 1990. Unique features of sensilla on the antennae of Formicidae (Hymenoptera). Applied Entomology and Zoology 25: 491-501.
- Hisasue, Y. 2018. Ant fauna of Matsuyama Castle. ARI 39: 18-36.
- Hisasue, Y. 2020. A checklist of the ants of Mt. Hiko-san (Kyushu, Japan). Korasana 93: 31-38.
- Hisasue, Y., Tsuji, Y. 2020. Records of Nylanderia amia (Forel) in Shikoku, with notes of range expansion of Japan. ARI 41: 18-36.
- Ito, F., Makita, S., Nakao, H., Hosokawa, R., Kikuchi, T., Yamane, S. 2021. Thelytokous parthenogenesis by dealate queens in the myrmicine ant Monomorium hiten distributed in Nansei Islands, western Japan, with description of the male. Asian Myrmecology 14: e014001 (doi:10.20362@am.014001).
- Itow, T., Kobayashi, K., Kubota, M., Ogata, K., Imai, H.T., Crozier, R.H. 1984. The reproductive cycle of the queenless ant Pristomyrmex pungens. Insectes Sociaux 31, 87–102.
- Iwata, K., Eguchi, K., Yamane, S. 2005. A case study on urban ant fauna of southern Kyusyu, Japan, with notes on a new monitoring protocol (Insecta, Hymenoptera, Formicidae). Journal of Asia-Pacific Entomology 8, 263-272.
- Khachonpisitsak, S., Yamane, S., Sriwichai, P., Jaitrong, W. 2020. An updated checklist of the ants of Thailand (Hymenoptera, Formicidae). ZooKeys 998, 1–182 (doi:10.3897/zookeys.998.54902).
- Lee, C.-C., Weng, Y.-M., Lai, L.-C., Suarez, A.V., Wu, W.-J., Lin, C.-C., Yang, C.-C.S. 2020. Analysis of recent interception records reveals frequent transport of arboreal ants and potential predictors for ant invasion in Taiwan. Insects 11, 356 (doi:10.3390/INSECTS11060356).
- Mayr, G. 1886c. Notizen über die Formiciden-Sammlung des British Museum in London. Verh. K-K. Zool.-Bot. Ges. Wien 36: 353-368 (page 361, Combination in Pristomyrmex)
- Nakabayashi, Y., Mochioka, Y., Tokuda, M., Ohshima, I. 2020. Mutualistic ants and parasitoid communities associated with a facultative myrmecophilous lycaenid, Arhopala japonica, and the effects of ant attendance on the avoidance of parasitism. Entomological Science 23, 233–244 (doi:10.1111/ENS.12417).
- Nakajima, T. 2018. Evaluation of ant diversity in urban areas and research on IPM. Japanese Journal of Environmental Zoology 29, 149–158.
- Park, J., Park, J. 2021. Complete mitochondrial genome of the gate-keeper ant Colobopsis nipponica (Wheeler, W.M., 1928) (Formicidae: Hymenoptera). Mitochondrial DNA Part B 6, 86–88 (doi:10.1080/23802359.2020.1845581).
- Park, J., Xi, H., Park, J. 2021. Complete mitochondrial genome of the acrobat ant Crematogaster teranishii Santschi, 1930 (Formicidae; Hymenoptera). Mitochondrial DNA Part B 6, 593–595 (doi:10.1080/23802359.2021.1875922).
- Park, S.-H., Hosoishi, S., Ogata, K. 2014. Long-term impacts of Argentine ant invasion of urban parks in Hiroshima, Japan. Journal of Ecology and Environment 37, 123–129 (doi:10.5141/ecoenv.2014.015).
- Park, S.-H., Hosoishi, S., Ogata, K., Kasuya, E. 2014. Changes of species diversity of ants over time: A case study in two urban parks. Journal of the Faculty of Agriculture, Kyushu University 59(1), 71–76.
- Park, S.-H., Hosoishi, S., Ogata, K., Kuboki, Y. 2014. Clustering of ant communities and indicator species analysis using self-organizing maps. Comptes Rendus Biologies 337, 545–552 (doi:10.1016/j.crvi.2014.07.003).
- Saito-Morooka, F., Fukuhara, K., Suda, K. 2015. The ant fauna of Rissho University at Kumagaya, Saitama Prefecture (Insecta, Hymenoptera, Formicidae). Bulletin of geo-environmental science (17), 35-39.
- Satow, S., Satoh, T. & Hirota, T. 2013. Colony fusion in a parthenogenetic ant, Pristomyrmex punctatus. Journal of Insect Science 13:38.
- Siddiqui, J. A., Li, J., Zou, X., Bodlah, I., Huang, X. 2019. Meta-analysis of the global diversity and spatial patterns of aphid-ant mutualistic relationships. Applied Ecology and Environmental Research 17: 5471-5524 (doi:10.15666/aeer/1703_54715524).
- Smith, F. 1860b. Catalogue of hymenopterous insects collected by Mr. A. R. Wallace in the islands of Bachian, Kaisaa, Amboyna, Gilolo, and at Dory in New Guinea. J. Proc. Linn. Soc. Lond. Zool. 5(17b)(suppl. to vol. 4 4: 93-143 (page 108, worker described)
- Tanaka, K., Tokuda, M. 2020. Negative correlation between dispersal investment and canopy openness among populations of the ant-dispersed sedge, Carex lanceolata. Plant Ecology. (doi:10.1007/S11258-020-01065-6).
- Terayama, M., Sunamura, E., Fujimaki, R., Ono, T., Eguchi, K. 2021. A Surprisingly Non-attractiveness of Commercial Poison Baits to Newly Established Population of White-Footed Ant, Technomyrmex brunneus (Hymenoptera: Formicidae), in a Remote Island of Japan. Sociobiology 68, 5898 (doi:10.13102/sociobiology.v68i1.5898).
- Toyama, Y., Kuroki, I., Nakamura, K. 2021. Dispersal of Phraortes illepidus (Phasmida: Phasmatidae) eggs by workers of the queenless ant, Pristomyrmex punctatus (Hymenoptera: Formicidae). Sociobiology, 68(4), e7194 (doi:10.13102/sociobiology.v68i4.7194).
- Trible, W., Kronauer, D.J.C. 2017. Caste development and evolution in ants: it's all about size. Journal of Experimental Biology 220, 53–62 (doi:10.1242/jeb.145292).
- Tsuji, K. & Dobata, S. 2011. Social cancer and the biology of the clonal ant Pristomyrmex punctatus (Hymenoptera: Formicidae). Myrmecological News, 15, 91-99.
- Tsuji, K. 1988. Nest relocations in the Japanese queenless ant Pristomyrmex pungens Mayr. (Hymenoptera: Formicidae). Insect. Soc. 35:321-340.*Wang, C., Sung, P.-J., Lin, C.-C., Ito, F., Billen, J. 2023. Parthenogenetic reproduction in Strumigenys ants: An update. Insects 14, 195 (doi:10.3390/insects14020195).
- Wang, M. 2003. A Monographic Revision of the Ant Genus Pristomyrmex (Hymenoptera:Formicidae). Bulletin of the Museum of Comparative Zoology 157(6): 383-542 (page 412, fig. 92 Ergatoid queen, male described; page 410, Senior synonym of pungens)
- Wang, W.Y., Soh, E.J.Y., Yong, G.W.J., Wong, M.K.L., Benoit Guénard, Economo, E.P., Yamane, S. 2022. Remarkable diversity in a little red dot: a comprehensive checklist of known ant species in Singapore (Hymenoptera: Formicidae) with notes on ecology and taxonomy. Asian Myrmecology 15: e015006 (doi:10.20362/am.015006).
- Xiong, Z., He, D., Guang, X., Li, Q. 2023. Novel tRNA gene rearrangements in the mitochondrial genomes of poneroid ants and phylogenetic implication of Paraponerinae (Hymenoptera: Formicidae). Life 1310, 2068 (doi:10.3390/life13102068).
- Yamada, A., Eguchi, K. 2016. Description of the male genitalia of Pristomyrmex punctatus (Smith, 1860) (Hymenoptera, Formicidae, Myrmicinae). Asian Myrmecology 8: 1-8 (DOI:DOI: 10.20362/am.008010).
- Yu, Y. 2016. Risk of alien species introduction to Ogasawara Islands : Case study of ants at Tokyo Port. World Heritage Studies 1, 86-89.
- Zhu, Y. & Wang, D. (2014). Seed dispersal of Corydalis wilfordii and C. racemosa (Papaveraceae): effect of ant foraging and behavior and seed characteristics. Acta Ecologica Sinica, 34: 4938-4942. (In Chinese with English abstract). doi:10.5846/stxb201301010005
- Zhu, Y. and D. Wang. 2018. Leaf Volatiles from Two Corydalis Species Lure a Keystone Seed-dispersing Ant and Enhance Seed Retrieval. Sociobiology. 65:370-374. doi:10.13102/sociobiology.v65i3.2726
References based on Global Ant Biodiversity Informatics
- Abe T. 1974. Notes on the fauna of ants in Iriomote Island. Ecol. Stud. Nat. Cons. Ryukyu Isl. 1:105-111.
- Abe T. 1977. A preliminary study on the ant fauna of the Tokara Islands and Amami-Oshima. Ecol. Stud. Nat. Cons. Ryukyu Isl. 3: 93-102.
- Abe T., and A. Maeda. 1977. Fauna and density of ants in sugarcane fields of the southern part of Okinawa Island. Pp. 75-91 in: Ikehara, S. (ed.) 1977. Ecological studies of nature conservation of the Ryukyu Islands - (III). Naha, Okinawa: University of the Ryukyus, 202 pp.
- Anh L. N., K. Ogata, and S. Hosoichi. 2010. Ants of agricultural fields in Vietnam (Hymenoptera: Formicidae). Bull. Inst. Trop. Agr., Kyushu Univ. 33: 1-11.
- Azuma M. 1953. On the myrmecological fauna of Mt. Rokko, Hyogo Prefecture. Warera 2:1-7.
- Azuma M. 1977. On the myrmecological-fauna of Mt. Rokko, Hyogo, with description of a new species (Formicidae, Hymenoptera). Hyogo Biology 7:112-118.
- Azuma, S. and M. Kinjo. 1987. Family Formicidae, In Checklist of the insects of Okinawa. The Biological Society of Okinawa, Nishihara. Pages 310-312.
- CSIRO Collection
- Chapman, J. W., and Capco, S. R. 1951. Check list of the ants (Hymenoptera: Formicidae) of Asia. Monogr. Inst. Sci. Technol. Manila 1: 1-327
- Chen Y. Q., Q. Li, Y. L. Chen, Z. X. Lu, X. Y. Zhou. 2011. Ant diversity and bio-indicators in land management of lac insect agroecosystem in Southwestern China. Biodivers. Conserv. 20: 3017-3038.
- Chen Y., C. W. Luo, H. W. Li, Y. J. Liu, H. F. Zheng, and F. C. Yang. 2013. Investigation of ant species and distribution on Wuliang Mountain. Journal of Henan Agricultural Sciences 42(5): 118-122.
- Cheng D., Z. Chen, and S. Zhou. 2015. An analysis on the ant fauna of Jinzhongshan Nature Reserve in Gunagxi, China. Journal of Guangxi Normal University: Natural Science Edition 33(3): 129.137.
- Choi B.-M. 1987. Taxonomic study on ants (Formicidae) in Korea (1). On the genus Monomorium. Journal of the Institute of Science Education (Cheongju National Teachers' College) 11:17-30.
- Choi B.M. 1985. Study on distribution of ants (Formicidae) from Korea (1). Formic fauna in Mt. Songni. Cheongju Sabom Taehak Nonmunjip (Journal of Cheongju National Teachers' College) 22:401-437.
- Choi B.M. 1986. Studies on the distribution of ants (Formicidae) in Korea. Journal of Chongju National Teacher College 23: 317-386.
- Choi B.M. 1988. Studies on the distribution of ants (Formicidae) in Korea (5) Ant fauna in Is. Kanghwado. Chongju Sabom Taehak Nonmunjip (Journal of Chongju National Teacher' College) 25: 217-231.
- Choi B.M. 1996. Distribution of ants (Formicidae) in Korea (16) - Ant fauna from Chollabukdo. Korean J. Soil. Zoology 1(1): 5-23.
- Choi B.M. 1996. Distribution of ants (Formicidae) in Korea (16): Ant fauna from Chollabukdo. Korean Journal of Soil Zoology 1(1): 5-23.
- Choi B.M. 1996. Studies on the distribution of ants (Formicidae) in Korea (15) -Ant fauna islands Ullungdo and Dokdo. Journal of Chongju National University of Education 33: 201-219.
- Choi B.M. 1997. Distribution of Ants (Formicidae) in Korea (18). Ants Fauna in island Paekryongdo and Taechongdo. Journal of Chongju National University of Education 34: 119-138.
- Choi B.M. and C.H. Kim, 1987, Studies on the distribution of ants (Formicidae) in Korea (4). Ant fauna in Is. Hongdo and Is. Taehukusando. Journal of Chongju National Teacher College 24: 357-370.
- Choi B.M., Bang, J.R. 1992. Studies on the distribution of ants (Formicidae) in Korea (9). Ant fauna in Mt. Togyusan. Korean Journal of Applied Entomology 31:101-112.
- Choi B.M., I. H. Lee. 1995. Studies on the distribution of ants (Formicidae) in Korea (14). Ant fauna in island Sohuksando. Korean Journal of Applied Entomology 34(3): 191-197.
- Choi B.M., K. Ogata, and M. Terayama. 1993. Comparative studies of ant faunas of Korea and Japan. 1. Faunal comparison among islands of Southern Korean and northern Kyushu, Japan. Bull. Biogeogr. Soc. Japan 48(1): 37-49.
- Choi B.M., Kim, C.H., Bang, J.R. 1993. Studies on the distribution of ants (Formicidae) in Korea (13). A checklist of ants from each province (Do), with taxonomic notes. Cheongju Sabom Taehakkyo Nonmunjip (Journal of Cheongju National University of Education) 30: 331-380.
- Choi B.M., and H.S. Lee. 1999. Studies on the distribution ants in Korea (21) - Ant fauna in Kwanaksan. Korean J. Soil Zoology 4(1): 1-4.
- Choi B.M., and J. R. Bang. Studies on the distribution of ants (Formicidae) in Korea (12): the analysis of ant communities in 23 islands. Journal of Cheongju National University of Education 30:317-330.
- Clouse R. M. 2007. The ants of Micronesia (Hymenoptera: Formicidae). Micronesica. 39: 171-295.
- Collingwood C. A. 1962. Some ants (Hym. Formicidae) from north-east Asia. Entomologisk Tidskrift 83: 215-230.
- Collingwood C. A. 1976. Ants (Hymenoptera: Formicidae) from North Korea. Annales Historico-Naturales Musei Nationalis Hungarici 68:
- Collingwood C. A. 1981. Ants (Hymenoptera: Formicidae) from Korea, 2. Folia Entomologica Hungarica 42(34): 25-30.
- Dias R. K. S. 2002. Current knowledge on ants of Sri Lanka. ANeT Newsletter 4: 17- 21.
- Eguchi K., and S. Yamane. 2003. Species diversity of ants (Hymenoptera, Formicidae) in a lowland rainforest, northwestern Borneo. New Entomol. 52(1,2): 49-59.
- Eguchi K.; Bui T. V.; Yamane S. 2011. Generic synopsis of the Formicidae of Vietnam (Insecta: Hymenoptera), part I Myrmicinae and Pseudomyrmecinae. Zootaxa 2878: 1-61.
- Eto S., and K. Ogata. 1983. Ants of Hirado Island, Kyushu. Bulletin of the Nagasaki Prefecture Biological Group 25: 7-11.
- Forel A. 1901. Variétés myrmécologiques. Annales de la Société Entomologique de Belgique 45: 334-382.
- Forel A. 1903. Les Formicides de l'Empire des Indes et de Ceylan. Part X. J. Bombay Nat. Hist. Soc. 14: 679-715.
- Forel A. 1911. Die Ameisen des K. Zoologischen Museums in München. Sitzungsber. Math.-Phys. Kl. K. Bayer. Akad. Wiss. Münch. 11: 249-303.
- Forel A. 1912. H. Sauter's Formosa-Ausbeute. Formicidae (Hym.) (Schluss). Entomol. Mitt. 1: 45-61.
- Forel A. 1913. H. Sauter's Formosa-Ausbeute: Formicidae II. Arch. Naturgesch. (A)79(6): 183-202
- Fukumoto S. 2017. Records of ants (Hymenoptera: Formicidae) from Uji-shima, Uji Islands, Kagoshima, Japan. Nature of Kagoshima 43: 295–296.
- Fukumoto S. and Sk. Yamane. 2015. Records of ants from Uke–jima, Amami Islands, Japan (Hymenoptera, Formicidae). Nature of Kagoshima 41: 195–197.
- Fukumoto S., Jaitrong W. and Yamane S.K. 2013. Ant Fauna of Kuro-shima, Iwo-jima and Take-shima islands, Kagoshima Prefecture, southwestern Japan. Nature of Kagoshima 39: 119-125
- Fukumoto S., R. Satria, T. Maeda, and S. Yamane. 2014. Ant fauna of Gaja-jima, Tokara Islands, southwestern Japan. Nature of Kagoshima 40: 127131.
- Fukumoto S., Sk. Yamane, and M. Hira. 2016. Records of ants from Yoro-Shima, Amami Gunto, Japan (Hymenoptera, Formicidae). Nature of Kagoshima 42: 461–464.
- Fukumoto S., W. Jaitrong, and S. Yamane. 2013. Ant fauna of Take-shima, Iwo-jima and Kuro-shima islands, Kagoshima Prefecture, southwestern Japan. Nature of Kagoshima 39: 99-105.
- Guo X., Q. Lin, J. Cui, D. Gao, S. Xu, and Z. Sheng. 2014. Biodiversity and spatial distribution patterns of ant species in tea gardens of Chongqing. Chinese Journal of Eco-Agriculture DOI: 10.3724/SP.J.1011.2014.31052
- Guénard B., and R. R. Dunn. 2012. A checklist of the ants of China. Zootaxa 3558: 1-77.
- Ha S.J, S.J. Park, and B.J. Kim. 2002. Comparative ant faunas between Seonyudo and seven other islands of West Sea in Korea. Korean Journal of Entomology 32(2): 75-79.
- Harada Y. 1997. Ants from the Koshiki islands, Kagoshima-ken, southern Japan. Ari 21: 1-4.
- Harada Y. 2000. Ant fauna of the forest floor of the Koshikijima Islands, Kagoshima-ken, southern Japan. Ari 24: 4-11.
- Harada Y. 2011. Arboreal ant fauna of Joyama Park, Kagoshima Prefecture, southern Japan. Asian Myrmecology 4: 79-87.
- Harada Y. S. Koto, N. Kawaguchi, K. Sato, T. Setoguchi, R. Muranaga, H. Yamashita, A. Yo, and S. Yamane. 2012. Ants of Jusso, Isa City, Kagoshima Prefecture, southwestern Japan. Bull. biogeogr. Soc. Japan 67: 143-152.
- Harada Y., D. Fukukura, R. Kurisu, and S. Yamane. 2013. Ants of Ports, monitoring of alien ant species. Bull. Biogeogr. Soc. Japan 68: 29-40.
- Harada Y., H. Yadori, M. Yoneda, R. Takinami, K. Nagahama, Y. Matsumoto, A. Oyama, S. Maeda, and S. Yamane. 2009. Ant fauna of Tanegashima (Hymenoptera, Formicidae). Nankiseibutu, the Nanki Biological Society 51(1): 15-21.
- Harada Y., K. Asai, M. Araba and T. Higasayama. 2018. Ants at ports on Kuro-shima, Iwo-jima and Take-shima, Kagoshima Prefecture, Japan. Nature of Kagoshima 45: 129–134.
- Harada Y., K. Asai, M. Araba, T. Higasayama, and N. Saito. 2019. Ant fauna at ports on the Goto Islands – monitoring of alien ant species –. Nature of Kagoshima 46: 27–32.
- Harada Y., K. Nishikubo, K. Matsumoto, M. Matsuda, Y. Inazawa, Y. Ozono, S. Koto, N. Kawaguchi, and S. Yamane. 2011. Ant fauna of Japanese beech (Fagus crenata) forests in southwestern Japan. Bull. Biogeogr. Soc. Japan 66: 115-127.
- Harada Y., K. Tashiro, K. Ebihara, H. Yadori, M. Yoneda, R. Takinami, K. Nagahama, and K. Hayashi. 2008. Ant fauna of the lavas of Sakurajima Volcano, Southern Japan. Bull. Biogeogr. Soc. Japan 63: 205-215.
- Harada Y., M. Enomoto, K. Nishimuta, and H. Mizumata. 2015. Ants of the Amami Islands, central Ryukyus, Japan. Nature of Kagoshima 41: 199–208.
- Harada Y., M. Enomoto, N. Nishimata, and K. Nishimuta. 2014. Ants of the Tokara Islands, northern Ryukyus, Japan. Nature of Kagoshima 40: 111121.
- Harada Y., S. Haruguchi, T. Iwasaki, K. Onishi, Y. Tashiro, and Sk Yamane. 2010. Ants from Japanese cherry trees, Prunus x yedoensis, in public parks in Kagoshima, southwestern Japan. Bull. Biogeogr. Soc. Japan 65: 169-179.
- Harada Y., T. Yamaguchi, D. Fukukura, and H, Mizumata. 2014. Ants of ports on Amami Islands - Monitoring of alien ant species. Bull. Biogeogr. Soc. Japan. 69: 83-90.
- Harada Y., Y. Matsumoto, S. Maeda, A. Oyama, and S. Yamane. 2009. Comparison of ant fauna among different habitats of Yaku-shima Island, southern Japan. Bull. Biogeogr. Soc. Japan 64: 125-134.
- Hasegawa M. S. Sugiura, M. T. Ito, A. Yamaki, K. Hamaguchi, T. Kishimoto, and I. Okochi. 2009. Community structures of soil animals and survival of land snails on an island of the Ogasawara Archipelago. Pesq. agropec. bras., Brasília 44(8): 896-903.
- Hiromori T. 2003. Insects recorded in August, 2002 in Akuseki-Is, Toshima-mura, Kagoshima Prefecture. Kagoshima Prefecture. Bull. Kagoshima Pref. Mus. 22: 75-82.
- Hosoichi S., M. M. Rahman, T. Murakami, S. H. Park, Y. Kuboki, and K. Ogata. 2019. Winter activity of ants in an urban area of western Japan. Sociobiology 66(3): 414-419.
- Hosoichi S., M. Yoshimura, Y. Kuboki, and K. Ogata. 2007. Ants from Yakushima Island, Kagoshima Prefecture. Ari 30: 47-54.
- Hosoichi S., W. Tasen, S. H. Park. A. Le Ngoc, Y. Kuboki, and K. Ogata. 2015. Annual fire resilience of ground-dwelling ant communities in Hiraodai Karst Plateau grassland in Japan. Entomological Science 18: 254–261.
- Hosoishi S. 2006. Ant fauna of Noko Island. pp99-107. In: The floristic and faunistic surveys of the Noko Island.
- Hosoishi S., M. Yoshimura, Y. Kuboki, and K. Ogata. 2007. Ants from Yakushima Island , Kagoshima Prefecture. Ari 30: 47-54.
- Hu C.-H. 2006. Indigenized conservation and biodiversity maintenance on Orchid Island. PhD Thesis, graduate school of the University of Minnesota. 150 pages.
- Ichikawa A. 1999. Records of ants observed from several localities of Osaka Prefecture, Japan, -1. Ari 23: 1-3.
- Ikeshita Y., A. Gotoh, K. Yamamoto, N. Taniguchi, and F. Ito. 2007. Ants collected in Mt. Linoyama, Marugame, Kagawa Prefecture (Hymenoptera, Formicidae). Kagawa Seibutsu 34: 59-62.
- Ikudome S. and S. Yamane. 2009. Ants, wasps and bees of Take-shima, Northern Ryukyus, Japan (Hymenoptera, Aculeata). Bull. Inst. Minami-Kyushu Reg. Sci. (Kagoshima Women's Jr. Coll.) 25: 1-8.
- Ito. F., Kondoh. M., Kubota. S., Masuko. K., Morishita. M., Murata. K., Ogata. K., Sato. T., Takamine. H., Yamaoka. H. and Kondoh. M. 1986. A list of ants collected at Akiyoshi-dai (Yamaguchi-ken) by the members of the Myrmecologists Society (Japan) in 1985. ARI Reports of the Myrmecologists Society (Japan) 14: 5-6
- Iwata, Kouki, Eguchi, Katsuyuki and Yamane, Seiki. 2005. A Case Study on Urban Ant Fauna of Southern Kyusyu, Japan, with Notes on a New Monitoring Protocol (Insecta, Hymenoptera, Formicidae). Asia-Pacific Entomol. 8(3):263-272.
- Jaitrong W., B. Guenard, E. P. Economo, N. Buddhakala, and S. Yamane. 2016. A checklist of known ant species of Laos (Hymenoptera: Formicidae). Asian Myrmecology 8: 1-32. DOI: 10.20362/am.008019
- Jaitrong W., and T. Ting-Nga. 2005. Ant fauna of Peninsular Botanical Garden (Khao Chong), Trang Province, Southern Thailand (Hymenoptera: Formicidae). The Thailand Natural History Museum Journal 1(2): 137-147.
- Jaitrong W.; Nabhitabhata, J. 2005. A list of known ant species of Thailand. The Thailand Natural History Museum Journal 1(1): 9-54.
- Janda M., G. D. Alpert, M. L. Borowiec, E. P. Economo, P. Klimes, E. Sarnat, and S. O. Shattuck. 2011. Cheklist of ants described and recorded from New Guinea and associated islands. Available on http://www.newguineants.org/. Accessed on 24th Feb. 2011.
- Katayama M., T. Hosoya, and W. Toki. 2013: First survey of ground-dwelling ants (Hymenoptera: Formicidae) on the uninhabitedGaja-jima Island, theRyukyu archipelago, Japan.Entomol. Fennica 24: 216222.
- Kawahara Y., S. Hosoyamada, and S. Yamane. 1999. Ant fauna of the Terayama Station for Education and Research on Nature, Kagoshima University. Bulletin of the Faculty of Education, Kagoshima University. Natural Science 50: 147-156.
- Kim B., Ryu D., Park S., and J. Kim. 1994. Systematic study on ants from coasts of Korean Peninsula (Hym: Formicidae). Korean journal of entomology 24: 293-309.
- Kim B.J. 1996. Synonymic list and distribution of Formicidae (Hymenoptera) in Korea. Entomological Research Bulletin Supplement 169-196.
- Kim B.J., K.G. Kim, D.P. Ryu, J.H. Kim. 1995. Ants of Chindo island in Korea (Hymenoptera; Formicidae). The Korean Journal of Systematic Zoology 11(1): 101-113.
- Kim B.J., K.G. Kim, J.H. Kim, S.J. Park. Ants from Mt. Mirok. Korean J. Soil Zoology 2(2): 115-128.
- Kim B.J., S.J. Park, and J.H. Kim. 1996. Ants from Naejangsan national park (Hymenoptera: Formicidae). Korean J. Soil. Zoology &(2): 120-133.
- Kim B.J.; Kim, K.G.; Lim, K.H. 1993. Systematic study of ants from Chejudo province. Korean Journal of Entomology 23: 117-67
- Kim B.J.; Kim, K.G.; Lim, K.H. 1993. Systematic study of ants from Chejudo province. Korean Journal of Entomology 23: 117-70
- Kim C.H., B.M. Choi, and J.R. Bang. 1992. Studies on the distribution of ants (Formicidae) in Korea (8)-Ant fauna in 10 islands, Chollanam-do. Korean J. Appl. Entomol. 31(4): 345-359.
- Kim K.I., C.H. Kim, and B. Choi. 1989. The ant fauna of the southern shore in Gyeongsangnamdo, Korea. Journal of Gyeongsang Nat. Univ. 28(2): 213-226.
- Kim et al. 1993. Systematic study of ants from Chejudo Province. Koran Journal of Entomology 23(3): 117-141.
- Kim, Byung-Jin, Ky-Gyong Kim, Dong-Pyo Ryu and Joong-Hyon Kim. 1995. The Korean Journal of Systematic Zoology. 11(1):101-113.
- Kishimoto R., and N. Tsurusaki. 2011. Ant fauna of Tottori Sand dunes and surrounding sand erosion control forests. Sanin Natural History Research 6: 37-44.
- Kondoh M., and Y. Kitazawa. 1984. Ant communities of the campus of UOEH and in an adjacent natural forest. Journal of UOEH 6(3): 221-234.
- Kuboki Y., K. Ogata, and E. Kasuya. 2006. Note on the bait trapping method. ANeT Newsletter 8: 16-19
- Kubota S., and M. Terayama. 1982. Ant fauna of Kanagawa Prefecture, Japan (IV) Ants of Kakio. Kanagawa-chuho (Journal of the Kanagawa Entomologists Association) 21-28.
- Kubota. S., and M. Terayama. 1988. Ant fauna of Tokyo. (1) A list of ants collected at the parks. ARI Reports of the Myrmecologists Society (Japan) 16: 14-16
- Kwon T. S. 2012. Korean ant atlas. Korea Forest Research Institute 162 pages.
- Kwon T. S. 2015. Ant assemblages along the Baekdudaegan Mountain Range in South Korea: Human roads and temperature niche. Journal of Asia-Pacific Biodiversity 8: 152-157.
- Kwon T.S., C. M. Lee, J. H. Chun, J. H. Sung, and S. K. Kim. 2011. Ants in Hongneung forest. Korea Forest Research Institute, 92 pages.
- Lee T. L., and Y. S. Wei. 2005. Study for the temporal and spatial variation of the ant assemblages as the biological indicator in national parks. Journal of Animal and Veterinary Advances 4(4): 491-496.
- Li Q., Y. Chen, S. Wang, Y. Zheng, Y. Zhu, and S. Wang. 2009. Diversity of ants in subtropical evergreen broadleaved forest in Pu'er City, Yunnan. Biodiversity Science 17(3): 233-239.
- Li X., D. Hao, and Y. Huang. 2011. Ant species diversity at piedmont of Zijin Mountain in Nanjing. Journal of Nanjing Forestry University ( Natural Science Edition) 35(5): 55-58.
- Li Z.h. 2006. List of Chinese Insects. Volume 4. Sun Yat-sen University Press
- Lin C. C., and W. J. Wu. 1998. The ant tribe Myrmecinini (Hymenoptera: Formicidae) of Taiwan. Chinese Journal of Entomology 18: 83-100.
- Lin S. Y., B. Di Giusto, A. Bain, and L. S. Chou. 2016. Variation of ant community structure on Ficus benguetensis. Taiwania 61(1): 49-57.
- Lyu D.P. 2003. Systematics of Myrmicinae from Korea (Hymenoptera: Formicidae). PhD thesis Faculty of the Graduate School of Chungbuk National University 330 pages.
- Maeto K. and S. Sato. 2004. Impacts of forestry on ant species richness and composition in warm-temperate forests of Japan. Forest Ecology and Management 187: 213223.
- Manabe K. 1994. Ants of the shrine forest in Fukuoka Prefecture (First report; Ants of lowland). Ari 17: 6.
- Manabe K. 1994. Ants of the shrine forest in Fukuoka Prefecture (first report; ants of lowland). Ari 18: 6.
- Manabe K. 1994. Ants of the shrine forest in Fukuoka Prefecture (second report; ants of mountain zone). Ari 17: 8.
- Mano T., and N. Kanie. 2004. Investigations of insects at Hirosawagawa river in the northern area of Toyota City (Aichi Prefecture). Yahagi research (?????) 8: 123-147.
- Masuko K. 1982. A data on the myrmecofauna of Miyake Island. Ari 10: 1-2.
- Matsumura S. and Yamane Sk. 2012. Species composition and dominant species of ants in Jigenji Park, Kagoshima City, Japan. Nature of Kagoshima 38: 99107
- Matsumura S., and S. Yamane. 2012. Species composition and dominant species of ants in Jigenji Park, Kagoshima City, Japan. Nature of Kagoshima 38: 99-107.
- Minato M., T. Kameyama, F. Ito, and T. Itino. 1996. A preliminary report of ant fauna in Gagawa Prefecture. Ari 20: 9-13.
- Mizota K. 2002. A check list of insects in Kinkazan Island, Miyagi Pref., Northeastern Japan: A bibliographical Survey. Bulletin of Miyagi University of Education Environmental Education 5: 69-78.
- Morikawa K. 1957. Terrestrial fauna of Kashima islets in the bay of Tanabe, Wakayama Prefecture. Publications of the Seto Marine Biological Laboratory 6(2): 225-240.
- Morisita, M. 1941. Notes on Camponotus herculeanus subsp. vagus var. yessensis Teranishi (Hymenoptera, Formicidae). [In Japanese.]. Mushi 13:95. [1941-02-28] PDF 127445
- Ngoc Anh L., K. Ogata, and S. Hosoishi. 2010. Ants of agricultural fields in Vietnam (Hymenoptera: Formicidae). Bull. Inst. Trop. Agr. Kyushu Univ. 33: 1-11.
- Ochi K. 1983. Distribution pattern of ants in pine stands, with special reference to Monomorium nipponense Wheeler (Hymenoptera: Formicidae). Gensei 44:1-6.
- Ogata K. 1981. The ant fauna of the Goto islands, Natural history of the Goto? Islands, Japan : Iki Tsushima to no taihi (Danjo Gunto? Ko?rai Sone o fukumu Japan: 347-351.
- Ogata K., Y. Hirashima, T. Miura, Y. Maeta, K. Yano, and J. Ko. 1985. Ants collected in pine forests infested by the pine needle gall midge in Korea (Hymenoptera, Formicidae). Esakia (23): 159-163.
- Ogata. K., Touyama, Y. and Choi, B. M. 1994. Ant fauna of Hiroshima Prefecture, Japan. ARI Reports of the Myrmecologists Society (Japan) 18: 18-25
- Onoyama K. 1976. A premilinary study on the ant fauna of Okinawa-ken, with taxonomic notes (Japan; Hymenoptera: Formicidae). Ecol. Stud. Nat. Cons. Ryukyu Isl. II: 121-141.
- Paik W.H. 1984. A checklist of Formicidae (Hymenoptera) of Korea. Korean J. Plant Prot. 23(3): 193-195.
- Park S. H., S. Hosoishi, K. Ogata, and Y. Kuboki. 2014. Clustering of ant communities and indicator species analysis using self-organizing maps. Comptes Rendus Biologies http://dx.doi.org/10.1016/j.crvi.2014.07.003
- Park S.J., and B.J. Kim. 2002. Faunal comparison of ants among Cheongsando and other islands of South Sea in Korea. Korean Journal of Entomology 32(1): 7-12.
- Park, Seong, Joon and Byung, and Kim, Jin. 2002. Faunal Comparison of Ants among Cheongsando and Other Islands of South Sea in Korea. Korean Jornal of Entomology. 32(1):7-12.
- Pfeiffer M.; Mezger, D.; Hosoishi, S.; Bakhtiar, E. Y.; Kohout, R. J. 2011. The Formicidae of Borneo (Insecta: Hymenoptera): a preliminary species list. Asian Myrmecology 4:9-58
- Pfeiffer, M., H. Cheng Tuck, and T. Chong Lay. 2008. Exploring arboreal ant community composition and co-ccurrence patterns in plantations of oil palm Elaeis guineensis in Borneo and Peninsular Malaysia. Ecography 31(1): 21-32.
- Radchenko, A. 2005. Monographic revision of the ants (Hymenoptera: Formicidae) of North Korea. Annales Zoologici (Warsaw) 55: 127-221.
- Radchenko, A. 2005. Monographic revision of the ants (Hymenoptera, Formicidae) of North Korea. Annales Zoologici 55(2): 127-221.
- Santschi F. 1920. Fourmis d'Indo-Chine. Annales de la Société Entomologique de Belgique 60: 158-176.
- Santschi F. 1924. Fourmis d'Indochine. Opuscules de l'Institut Scientifique de l'Indochine 3: 95-117
- Santschi F. 1925. Contribution à la faune myrmécologique de la Chine. Bulletin de la Société Vaudoise des Sciences Naturelles 56: 81-96.
- Sato T., N. Tsurusaki, K. Hamaguchi, and K. Kinomura. 2010. Ant fauna of Tottori prefecture, Honshu, Japan. Bulletin of the Tottori Prefectural Museum 47: 27-44.
- Shimana Y., and S. Yamane. 2009. Geogrpahical distribution of Technomyrmex brunneus Forel (Hymenoptera, Formicidae) in the western part of the mainland of Kagoshima, South Kyushu, Japan. Ari 32: 9-19.
- Shimazaki, A. and Miyashita, T. 2005. Variable dependence on detrital and grazing food webs by generalist predators: aerial insects and web spiders. Ecography 28: 485-495.
- Shimono A., and S. Yamane. 2003. Ant species diversity on Okinoerabu-jima, the Ryukyus, southern Japan. For the Establishment of Remote Islands Study (Kagoshima Univ.) 3: 11-29.
- Shindo, M. 1979. Ants of the Bonin Islands. Konshu to shizen 14(10): 24-28.
- Skarbek C. J., M. Noack, H. Bruelheide, W. Hardtle, G. von Oheimb, T. Scholten, S. Seitz, M. Staab. 2019. A tale of scale: plot but not neighbourhood tree diversity increases leaf litter ant diversity. Journal of Animal Ecology DOI: 10.1111/1365-2656.13115
- Smith, Fr. "Catalogue of hymenopterous insects collected by Mr. A. R. Wallace in the Islands of Bachian, Kaisaa, Amboyna, Gilolo, and at Dory in New Guinea." Journal of the Proceedings of the Linnean Society of London, Zoology 5 (1860): 93-143.
- Song Y., Z. Xu, C. Li, N. Zhang, L. Zhang, H. Jiang, and F. Mo. 2013. An Analysis on the Ant Fauna of the Nangun river Nature Reserve in Yunnan, China. Forest Research 26(6): 773-780.
- Staab M., A. Schuldt, T. Assmann, H. Bruelheide, and A.M. Klein. 2014. Ant community structure during forest succession in a subtropical forest in South-East China. Acta Oecologia 61: 32-40.
- Staab M., J. Methorst, J. Peters, N. Bluthgen, and A. M. Klein. 2017. Tree diversity and nectar composition affect arthropod visitors on extrafloral nectaries in a diversity experiment. Journal of Plant Ecology 10(1): 210-212.
- Staab M., N. Bluthgen, and A. M. Klein. 2014. Tree diversity alters the structure of a tri-trophic network in a biodiversity experiment. Oikos doi: 10.1111/oik.01723
- Sunamura E., K. Nishisue, M. Terayama, and S. Tatsuki. 2007. Invasion of Four Argentine Ant Supercolonies into Kobe Port, Japan: Their Distributions and Effects on Indigenous Ants (Hymenoptera: Formicidae). Sociobiology 50: 659-674.
- Suwabe, Mayuko, Ohnishi, Hitoshi, Kikuchi, Tomonori, Kawara, Kengo and Tsuji, Kazuki. 2009. Difference in seasonal activity pattern between non-native and native ants in subtropical forest of Okinawa Island, Japan. Ecol Res. 24:637-643.
- Takechi F. 1960. A list of ants unrecorded from Mt. Ishizuchi and Omogo Valley, Iyo, Shikoku (Hymenoptera: Formicidae). Transactions of the Shikoku Entomological Society 6:91.
- Tanaka B., N. Kanie, T. Mano, R. Arita, and A. Shiragane. 1999. The insect fauna between Takabashi bridge and Nomi-kouen Park, margins of the Yahagigawa river. ????? 3: 35-79.
- Terayama M. 1977. Checklist of the known ants of Saitama Prefecture. Insects and nature 12(4): 26-27
- Terayama M. 1981. Distribution of ants of the Nansei Archipelago. (I) Ants in the Amami Islands. Nature and Insects 16(8): 34-36.
- Terayama M. 1983. Kagoshima-ken-hondo no ari. Kanagawa-chucho (Journal of the Kanagawa Entomologists Association): 13-24.
- Terayama M. 1992. Structure of ant communities in East Asia. A. Regional differences and species richness. Bulletin of the Bio-geographical Society of Japan 47: 1-31.
- Terayama M. 1992. Structure of ant communities in east Asia. 1. Regional differences and species richness. Bull. Biogeogr. Soc. Japan 47(1): 1-31.
- Terayama M. 2005. Ants from the Tokiwamatsu Imperial villa, Tokyo. Mem. Natn. Sci. Mus., Tokyo 39: 245-247.
- Terayama M. 2009. A synopsis of the family Formicidae of Taiwan (Insecta: Hymenoptera). Research Bulletin of Kanto Gakuen University. Liberal Arts 17:81-266.
- Terayama M. and E. Hasegawa. 1991. Ant fauna of the Ogasawara islands. OgasawaraKenkyuNenpo 15: 4051.
- Terayama M., Choi, B.M., Kim, C.H. 1992. A check list of ants from Korea, with taxonomic notes. Bulletin of the Toho Gakuen 7:19-54.
- Terayama M., K. Ogata, and B.M. Choi. 1994. Distribution records of ants in 47 prefectures of Japan. Ari (report of the Myrmecologists Society of Japan) 18: 5-17.
- Terayama M., S. Kubota, and K. Eguchi. 2014. Encyclopedia of Japanese ants. Asakura Shoten: Tokyo, 278 pp.
- Terayama M., and K. Murata. 1990. Effects of area and fragmentation of forests for nature conservation: Analysis by ant communities. Bull. Biogeogr. Soc. Japan 45(2): 11-17.
- Terayama M., and S. Kubota. 1994. Ants from Aogashima Island. Ari 17: 11.
- Terayama M., and S. Kubota. 2002. Ants of Tokyo, Japan. ARI 26: 1-32.
- Terayama M., and S. Yamane. 1984. Ants of Yaku-shima Island, the northern Ryukyus, with reference to their altitudinal distribution (Insecta: Hymenoptera). Cons. Rep. Yaku-shima Wildness Area, Kyushu, Japan, pp. 643-667. Nat. Cons. Bureau, Env. Agency, Japan.
- Terayama, M. 2009. A synopsis of the family Formicidae of Taiwan (Insecta; Hymenoptera). The Research Bulletin of Kanto Gakuen University 17: 81-266.
- Terayama. M. 2004. Geological and ecological distribution of Japanese ants communities. (translated from Japanese) Reports of the Saitama Prefecture Animal Research Association. 48:31
- Teruyama. M. 1988. Ant fauna of Saitama Prefecture, Japan. ARI Reports of the Myrmecologists Society (Japan) 16: 4-13
- Touyama Y., N. Nakagoshi, and T. Yamamoto. 1997. Myrmecofauna of lucidophyllous forests in different developmental stages in south-western Japan. Ecological Research 12: 131-138.
- Viehmeyer H. 1912. Ameisen aus Deutsch Neuguinea gesammelt von Dr. O. Schlaginhaufen. Nebst einem Verzeichnisse der papuanischen Arten. Abhandlungen und Berichte des Königlichen Zoologischen und Anthropologische-Ethnographischen Museums zu Dresden 14: 1-26.
- Viehmeyer H. 1916. Ameisen von Singapore. Beobachtet und gesammelt von H. Overbeck. Archiv für Naturgeschichte (A)81(8):108-168.
- Wang C. and Wu J. 1992. Ants of the Jianfengling forest region in Hainan Province (Hymenoptera: Formicidae). Scientia Silvae Sinicae 28: 561-564.
- Wang M. 2003. A Monographic Revision of the Ant Genus Pristomyrmex (Hymenoptera:Formicidae). Bulletin of the Museum of Comparative Zoology 157(6): 383-542.
- Wang M. 2003. A monographic revision of the ant genus Pristomyrmex (Hymenoptera:Formicidae). Bulletin of the Museum of Comparative Zoology 157(6):383-542
- Wang M. 2003. A monographic revision of the ant genus Pristomyrmex (Hymenoptera: Formicidae). Bulletin of the Museum of Comparative Zoology 157(6): 383-542.
- Wang W. 2006. A taxonomic study of Formicidae ants from Hujiaping forest farm in Laifeng County. Journal of Hubei Institute for Nationalities (Natural Science Edition). 24(3): 298-300.
- Wheeler W. M. 1906. The ants of Japan. Bulletin of the American Museum of Natural History 22: 301-328.
- Wheeler W. M. 1921. Chinese ants. Bulletin of the Museum of Comparative Zoology 64: 529-547.
- Wheeler W. M. 1927. Chinese ants collected by Professor S. F. Light and Professor N. Gist Gee. American Museum Novitates 255: 1-12.
- Wheeler W. M. 1928. Ants collected by Professor F. Silvestri in China. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 22: 3-38.
- Wheeler W. M. 1928. Ants collected by Professor F. Silvestri in Japan and Korea. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 22: 96-125.
- Wheeler W. M. 1929. Ants collected by Professor F. Silvestri in Formosa, the Malay Peninsula and the Philippines. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 24: 27-64.
- Wheeler W. M. 1930. A list of the known Chinese ants. Peking Natural History Bulletin 5: 53-81.
- Wheeler W. M. 1930. Formosan ants collected by Dr. R. Takahashi. Proceedings of the New England Zoological Club 11: 93-106.
- Xu Z. H., B. L. Yang, and G. Hu. 1999. Formicidae ant communities in fragments of montane rain forest in Xishuangbanna, China. Zoological Research 20(4): 288-293.
- Xu Z. and Z. Zhang. 2002. Systematics of Chinese species of the ant genus Pristomyrmex Mayr (Hymenoptera: Formicidae). Entomologia Sinica 9(4): 69-72.
- Yamada A., and K. Eguchi. 2016. Description of the male genitalia of Pristomyrmex punctatus (Smith, 1860) (Hymenoptera, Formicidae, Myrmicinae). Asian Myrmecology 8: 1-6. DOI: 10.20362/am.008010
- Yamane S. 2016. How many species of Ants in Amami Islands? (in Japanese). Part 2, chapter 1 in How many species of Ants in Amami Islands? Pp. 92-132.
- Yamane S. 2019. Seasonal change in the foraging activity of ants in a residential area of mainland Kagoshima, Southwest Japan (Insecta, Hymenoptera, Formicidae). Nature of Kagoshima 45: 361–366.
- Yamane S. 2019. Seasonal change in the foraging activity of ants in a residential area of mainland Kagoshima, Southwest Japan (Insecta, Hymenoptera, Formicidae). Nature of Kagoshima 45: 361–366.
- Yamane S. S. Fukumoto, Y. Maeda, and Y. Sato. 2017. Records of ants from Kakeroma-jima, the Amami Islands, Japan. Bull. Biogeogr. Soc. Japan 71, 131-137.
- Yamane S., S. Ikudome, and M. Terayama. 1999. Identification guide to the Aculeata of the Nansei Islands, Japan. Sapporo: Hokkaido University Press, xii + 831 pp. pp, 138-317.
- Yamane S., Y. Harada and M. Yano. 1985. Ant fauna of Tanega-shima Island, the northern Ryukyus (Hymenoptera, Formicidae). Mem. Kagoshima Univ. Res. Center S. Pac. 6(1): 166-173.
- Yamane S., and S. Ikudome. 2008. Ants, wasps and bees of Kuro-shima Northern Ryukyus, Japan (Hymenoptera, Aculeata). Bull. Inst. Minami-Kyushu Reg. Sci. (Kagoshima Women's Jr. Coll.) 24: 1-9.
- Yamane S.; Bui T. V.; Ogata K.; Okido H.; Eguchi K. 2002. Ant fauna of Cuc Phuong National Park, North Vietnam (Hymenoptera: Formicidae). Bulletin of the Institute of Tropical Agriculture Kyushu University 25: 51-62.
- Yamane S.; Ikudome, S.; Terayama, M. 1999. Identification guide to the Aculeata of the Nansei Islands, Japan. Sapporo: Hokkaido University Press, xii + 831 pp. pp138-317.
- Yamane, S.; Iwai, T.; Watanabe, H.; Yamanouchi, Y. 1994. Ant fauna of the Tokara Islands, northern Ryukyus, Japan (Hymenoptera, Formicidae). WWF (Worldwide Fund for Nature) Japan Science Report 2(2):311-327.
- Yamazaki Y., S. Yamane, T. Hishida, T. Kuwahara, and N. Inoue. 2009. Ant fauna on the grounds of the Kashima-Jingu Shrine, Ibaraki, Central Japan (Hymenoptera, Formicidae). Bull. Ibaraki Nat. Mus. 12: 5-14.
- Yasuda M., and F. Koike. 2009. The contribution of the bark of isolated trees as habitat for ants in an urban landscape. Landscape and Urban Planning 92: 276-281.
- Yoshimura M. 2009. Impact of secondary forest management on ant assemblage composition in the temperate region in Japan. J. Insect. Conservation 13(5): 563-568.
- Yoshitomi H., and S. Matsuno. 2012. List of species of Hymenoptera and Diptera in Matsuyama City, Ehime Prefecture, Shikoku, Japan. pp. 167-176. In: Committee for Surveys of Natural Environment of Matsuyama City (Chief Editor: Kazuo ISHIKAWA) (ed.) Checklist of the Wild Animals, Fungi, and Plants of Matsuyama City, 2012. Published by the Department of Environment, Matsuyama City, 404 pp.
- Zettel, H. 2006. On the ants of the Philippine Islands: 1. The genus Pristomyrmex Mayr, 1866. Myrmecologische Nachrichten 8: 59-68.
- Zhang N. N., Y. Q. Chen, Z. X. Lu, W. Zhang, and K. L. Li. 2013. Species diversity, community structure difference and indicator species of leaf-litter ants in rubber plantations and secondary natural forests in Yunnan, southwestern China. Acta Entomologica Sinica 56(11): 1314-1323.
- Zhang R. J., L. W. Liang, and S. Y. Zhou. 2014. An analysis on the ant fauna of Nonggang Nature Reserve in Guangxi, China. Journal of Guangxi Normal university: Natural Science Edition 32(3): 86-93.
- Zhang W., and S. Zhou. 2016. An investigation on Formicidae species of Nanling National Park. Journal of Huizhou University 36(3): 27-30.
- Zhu C., K. Chi, X. Ye, H. Zhou, and C. Lio. 2010. Community structure and species diversity of ants in Xuzhou, Jiansu. Journal of Forestry Science and Technology 37(2): 7-10.
- Pages using DynamicPageList3 parser function
- Common Name
- Parthenogenetic
- Polygynous
- Photo Gallery
- North temperate
- North subtropical
- Tropical
- Aphid Associate
- Host of Aphis craccivora
- Host of Aphis gossypii
- Karyotype
- Species
- Extant species
- Formicidae
- Myrmicinae
- Crematogastrini
- Pristomyrmex
- Pristomyrmex punctatus
- Myrmicinae species
- Crematogastrini species
- Pristomyrmex species
- Ssr