Rogeria scobinata
| Rogeria scobinata | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Solenopsidini |
| Genus: | Rogeria |
| Species: | R. scobinata |
| Binomial name | |
| Rogeria scobinata Kugler, C., 1994 | |
All specimens were taken as strays in tropical forest below 1000 m, mostly by Berlese or Winkler sampling of leaf litter, rotten wood, or moss.
Identification
Kugler (1994) - creightoni species group. As in Rogeria alzatei, except the following: Clypeal apron truncate (none emarginate). MHI 0.96- 1.14. Posterior head with tuberculate macrosculpture. Erect hair usually absent from head dorsum; if present, it is short and usually confined to posterior margin.
Rogeria alzatei is a sibling species of scobinata, with which it is sympatric in Peru, Brazil, and Paraguay, but can be distinguished by characters in the diagnosis and key. The pair of columnar tubercles on the pygidium may also be distinctive.
Keys including this Species
Distribution
Rogeria scobinata ranges from the north coast of South America to Paraguay at elevations below 1000m.
Latitudinal Distribution Pattern
Latitudinal Range: 25.68015° to -25.509722°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Argentina, Bolivia, Brazil, Colombia, Ecuador, Guadeloupe, Guyana, Paraguay, Peru (type locality), Trinidad and Tobago.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
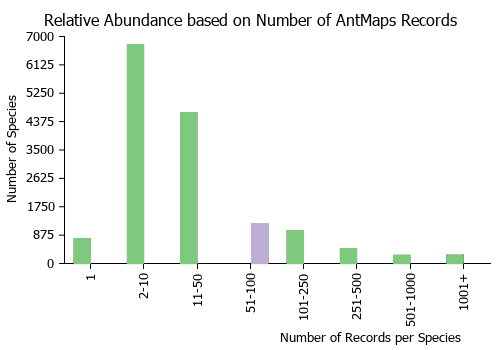
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
|
Biology
The following is modified from Kugler (1994): Little is known about these cryptic ants. Collection records typically range from sea level to 1000m, but five species extend higher and two (Rogeria unguispina and Rogeria merenbergiana) can be found at 2000m. Rogeria are generally collected in moist forests (primary or secondary forests, coffee or cacao plantations), but at higher elevations can be found in pastures (Rogeria leptonana, Rogeria merenbergiana). Several species (Rogeria creightoni, Rogeria cuneola, Rogeria foreli) have been found in moist and dry climates. Rogeria foreli is the most unusual, with some members dwelling at over 1800m in the temperate mountains of southern Arizona.
Most species have only been collected as strays or by Berlese or Winkler sampling, from leaf litter and rotten wood, but occasionally among epiphytes and moss (Rogeria belti, creightoni, Rogeria exsulans). Nests of several species (belti, Rogeria blanda, merenbergiana) have been found under the loose bark of rotten logs. Nests of blanda and Rogeria tonduzi have been taken from the trunks of cacao trees. A nest of Rogeria leptonana was found at 1750m under a rock in a pasture.
Nests are rarely found. Males are known for only four species (belti, blanda, leptonana and Rogeria stigmatica) and queens associated through nest series for only nine species.
Castes
Males have not been collected.
Queen
Images from AntWeb
   
| |
| Queen (alate/dealate). Specimen code casent0178170. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ALWC, Alex L. Wild Collection. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- scobinata. Rogeria scobinata Kugler, C. 1994: 54, figs. 61-62, 100 (w.q.) PERU.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Holotype and Paratype. TL 1.9-2.5 (2.2), HL 0.50-0.60 (0.55), HW 0.42-0.52 (0.475), SL 0.31-0.40 (0.35), EL 0.06-0.09 (0.07) (10-14 facets), PW 0.30-0.40 (0.35), WL 0.50-0.67 (0.585), SpL 0.08-0.12 (0.095), PetL 0.19-0.26 (0.23), PpetL 0.12-0.16 (0.14)mm, CI 0.82-0.86 (0.86), OI 0.14-0.18 (0.15), SI 0.74-0.78 (0.74), PSI 0.16-0.19 (0.16), MHI 0.96-1.09 (1.00). N=5
Nontypes. TL 1.9-2.5, HL 0.50-0.61, HW 0.44-0.52, SL 0.31-0.40, EL 0.06-0.09 (7-15 facets), PW 0.30-0.40, WL 0.51-0.68, SpL 0.08-0.13, PetL 0.19-0.28, PpetL 0.12-0.17mm, CI 0.84-0.85, OI 0.14-0.18, SI 0.70-0.78, PSI 0.16-0.20, MHI 1.00-1.14. N=32
Mandibles sub triangular, 5-toothed (sometimes with 1-2 additional basal denticles), decreasing in size basad; basal tooth small. Palpal formula 2,2. Median clypeus of some nontype workers from Colombia like that of alzatei, but type specimens with less prominent corners and other nontypes (Bolivia, some Brazil) have an almost evenly convex clypeal apron. Body of clypeus not projecting over clypeal apron. Posterior outline of head weakly concave medially to weakly convex. Nuchal groove clearly visible in side view. Eye oval to elliptical. Anterior and dorsal faces of pronotum may join smoothly, or in a weak angle. Metanotal groove broad, slightly less to slightly more impressed than shown in Fig. 61, bordered behind by a transverse carina. Propodeal spines inclined. Metapleural lobes moderately prominent; corner varies from sharply angular (Ecuador, some Peru) to rounded as in Fig. 44 (some Paraguay). Ventral petiolar peduncle usually with a weak, nonlamellate keel, but some Ecuadorian specimens with distinct keel. Postpetiolar node in dorsal view subrectangular as in Fig. 58. Pygidium with a pair of median, columnar, piligerous tubercles near caudal edge (barely visible in dissection microscope at 50X).
Laterodorsa and sides of head densely areolate. Posterior head with short triangular, blunt tubercles in more or less distinct rows. Tuberculate sculpture usually extends across posterior quarter of head, but in a few specimens from Leticia, Benjamin Constant, and Paraguay, the ridges between the tubercles are not always completely lost, so the posterior head appears mostly fragmented-rugose, with only a few of the triangular tubercles. Interstices on most of head somewhat dulled by indistinct areolate icrosculpture, but smoother and quite shiny between tubercles on back of head; sides sometimes rather strongly microareolate. Anterior edge of pronotal disc with 1-4 more or less transverse rugae. Rest of promesonotum longitudinally rugose with frequent incomplete lateral spurs. Mesosoma sides weakly and sparsely rugose to rugose-areolate, but more strongly microareolate than on pronotal disc. Dorsal face of propodeum usually lacking macro sculpture, but rather strongly microareolate. Rest of mesosoma with indistinct microareolate sculpture. Petiolar node with broken vestigial macrosculpture. Postpetiole without macrosculpture; nearly smooth on top. Sides of nodes with weakmicroareolate sculpture that imparts a granular appearance; microsculpture usually weaker on postpetiole.
Workers from Leticia have 8-10 erect hairs along posterior rim of head and those from Benjamin Constant have sparse, short erect hairs on the posterior rim and along the midline.
Color brown to golden brown. Legs and antennae generally lighter than rest of body; gaster sometimes darker.
Queen
TL 2.4-2.6, HL 0.55-0.58, HW 0.46-0.52, SL 0.34-0.38, EL 0.1 1-0.13, PW 0.41-0.45, WL 0.68-0.74, SpL 0.1 1-0.14, PetL O.25-0.27, PpetL 0.14-0.16mm, CI 0.84-0.90, SI 0.70-0.75, PSI 0.16-0.19, MHI 0.64-0.69. N=7
Habitus shown in Fig. 62. Parapsidal furrows indistinguishable from grooves in sculpture. Anterior pronotum transversely rugose to rugoseareolate, becoming longitudinal on sides. Mesoscutum with longitudinal, often diverging rugae; mesoscutellum rugose or rugose-areolate.
Type Material
Holotype locality. PERU: Madre de Dios Department, 3km N Puerto Maldonado, 260m, primary forest remnant by side of road, berlesate of leaf litter and rotten wood, 13-16-VI-1981 (c. Kugler and R. R. Lambert) Museum of Comparative Zoology.
Paratype localities. PERU: 2 workers, 1 queen, holotype locality [MCZ]; 22 workers, 2 queens, 5km E Puerto Maldonado on Río Tambopata, Finca Medina, 260m, primary forest berlesate, 13-16-VI-1981 (c. Kugler and R. R. Lambert) [mouthparts, sting, 1 worker coated for SEM] The Natural History Museum, Charles Kugler Collection, Los Angeles County Museum of Natural History, MCZ, Museu de Zoologia da Universidade de Sao Paulo, Musee d'Histoire Naturelle Genève, National Museum of Natural History.
Etymology
The name scobinata, meaning having the nature of a rasp, refers to the sculpture on the posterior head, which has rows of teeth like a rasp.
References
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Conceição-Neto, R., França, E.C.B., Feitosa, R.M., Queiroz, J.M. 2021. Revisiting the ideas of trees as templates and the competition paradigm in pairwise analyses of ground-dwelling ant species occurrences in a tropical forest. Revista Brasileira de Entomologia 65, e20200026 (doi:10.1590/1806-9665-rbent-2020-0026).
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- Kugler, C. 1994. A revision of the ant genus Rogeria with description of the sting apparatus (Hymenoptera: Formicidae). J. Hym. Res. 3:17-89. (page 54, figs. 61-62, 100 worker, queen described)
- Ladino, N., Feitosa, R.M. 2022. Ants (Hymenoptera: Formicidae) of the Parque Estadual São Camilo, an isolated Atlantic Forest remnant in western Paraná, Brazil. ZOOLOGIA 39: e22001 (doi:10.1590/S1984-4689.v39.e22001).
- LaPolla, J. S. and J. Sosa-Calvo. 2006. Review of the ant genus Rogeria (Hymenoptera: Formicidae) in Guyana. Zootaxa. 1330:59-68.
- Meurgey, F. 2020. Challenging the Wallacean shortfall: A total assessment of insect diversity on Guadeloupe (French West Indies), a checklist and bibliography. Insecta Mundi 786: 1–183.
References based on Global Ant Biodiversity Informatics
- Franco W., N. Ladino, J. H. C. Delabie, A. Dejean, J. Orivel, M. Fichaux, S. Groc, M. Leponce, and R. M. Feitosa. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5): 509-543.
- Galkowski C. 2016. New data on the ants from the Guadeloupe (Hymenoptera, Formicidae). Bull. Soc. Linn. Bordeaux 151, 44(1): 25-36.
- Groc S., J. H. C. Delabie, F. Fernandez, M. Leponce, J. Orivel, R. Silvestre, Heraldo L. Vasconcelos, and A. Dejean. 2013. Leaf-litter ant communities (Hymenoptera: Formicidae) in a pristine Guianese rainforest: stable functional structure versus high species turnover. Myrmecological News 19: 43-51.
- Groc S., J. Orivel, A. Dejean, J. Martin, M. Etienne, B. Corbara, and J. H. C. Delabie. 2009. Baseline study of the leaf-litter ant fauna in a French Guianese forest. Insect Conservation and Diversity 2: 183-193.
- Kugler C. 1994. A revision of the ant genus Rogeria with description of the sting apparatus (Hymenoptera: Formicidae). Journal of Hymenoptera Research 3: 17-89.
- Lapolla J. S., and J. Sosa-Calvo. 2006. Review of the ant genus Rogeria (Hymenoptera: Formicidae) in Guyana. Zootaxa 1330: 59-68.
- Lapolla, J. S., and B. L. Fisher. "Review of the ant genus Rogeria (Hymenoptera: Formicidae) in Guyana." Zootaxa 1330 (2006): 59-68.
- Mertl A. L., J. F. A. Traniello, K. Ryder Wilkie, and R. Constantino. 2012. Associations of two ecologically significant social insect taxa in the litter of an amazonian rainforest: is there a relationship between ant and termite species richness? Psyche doi:10.1155/2012/312054
- Pires de Prado L., R. M. Feitosa, S. Pinzon Triana, J. A. Munoz Gutierrez, G. X. Rousseau, R. Alves Silva, G. M. Siqueira, C. L. Caldas dos Santos, F. Veras Silva, T. Sanches Ranzani da Silva, A. Casadei-Ferreira, R. Rosa da Silva, and J. Andrade-Silva. 2019. An overview of the ant fauna (Hymenoptera: Formicidae) of the state of Maranhao, Brazil. Pap. Avulsos Zool. 59: e20195938.
- Siqueira de Castro F., A. B. Gontijo, P. de Tarso Amorim Castro, and S. Pontes Ribeiro. 2012. Annual and Seasonal Changes in the Structure of Litter-Dwelling Ant Assemblages (Hymenoptera: Formicidae) in Atlantic Semideciduous Forests. Psyche doi:10.1155/2012/959715
- Vasconcelos, H.L., J.M.S. Vilhena, W.E. Magnusson and A.L.K.M. Albernaz. 2006. Long-term effects of forest fragmentation on Amazonian ant communities. Journal of Biogeography 33:1348-1356
- Wild, A. L. "A catalogue of the ants of Paraguay (Hymenoptera: Formicidae)." Zootaxa 1622 (2007): 1-55.


