Rogeria tonduzi
| Rogeria tonduzi | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Solenopsidini |
| Genus: | Rogeria |
| Species: | R. tonduzi |
| Binomial name | |
| Rogeria tonduzi Forel, 1899 | |
With the exception of one specimen from Guatemala, all Rogeria tonduzi specimens come from Costa Rica at elevations of 0-100m on both sides of the cordillera. Most specimens were collected by Jack Longino as strays on ground and vegetation. He found one worker in a Cyphomyrmex nest and another " ... on the base of a small tree, amongst some Pheidole workers" (unpublished field notes). Lyn Garling found a nest with a "tubular entrance with white 'fuzz'" in a cacao tree (field note on label). (Kugler 1994)
Identification
Kugler (1994) - Most similar to but not quite like other species in the scandens species group. WL 0.81-0.90mm. Eye large. Palpal formula 2,2. Propodeal spiracle faces laterally. Propodeal spines long. Postpetiolar sternum not enlarged. Posterior head with transversely arching rugae. Rugae on mesosoma and petiolar node thick and rounded. Decumbent hair abundant on head dorsum and legs; little on gaster; no decumbent or appressed hair on mesosoma or nodes. Scapes, head dorsum, mesosoma, nodes and gaster with abundant flexible, tapered, erect hair; none on extensor surfaces of legs. The following as in scandens-group diagnosis: metapleural lobes, petiole, pygidial gland sculpture, sting apparatus, and sculpture.
Rogeria belti occurs in the same localities and could be confused with Rogeria tonduzi, but belti has a distinct petiolar node and predominantly areolate pronotal sculpture. Rogeria lirata from northern South America is similar to tondllzi in size, clypeus, and sculpture, but lirata has smaller eyes (6-12 facets), a distinct petiolar node, and scapes with suberect hair only.
Keys including this Species
Distribution
Central America
Latitudinal Distribution Pattern
Latitudinal Range: 18.64° to -0.6364°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Costa Rica (type locality), Ecuador, Guatemala, Honduras, Mexico, Nicaragua.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
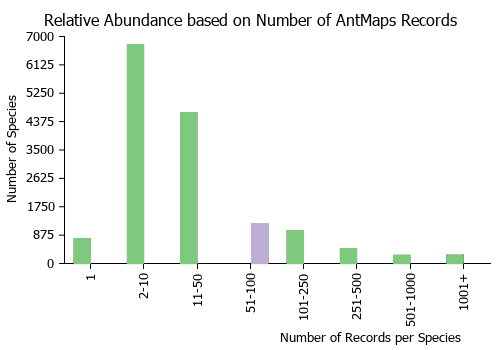
|
Biology
The following is modified from Kugler (1994): Little is known about these cryptic ants. Collection records typically range from sea level to 1000m, but five species extend higher and two (Rogeria unguispina and Rogeria merenbergiana) can be found at 2000m. Rogeria are generally collected in moist forests (primary or secondary forests, coffee or cacao plantations), but at higher elevations can be found in pastures (Rogeria leptonana, Rogeria merenbergiana). Several species (Rogeria creightoni, Rogeria cuneola, Rogeria foreli) have been found in moist and dry climates. Rogeria foreli is the most unusual, with some members dwelling at over 1800m in the temperate mountains of southern Arizona.
Most species have only been collected as strays or by Berlese or Winkler sampling, from leaf litter and rotten wood, but occasionally among epiphytes and moss (Rogeria belti, creightoni, Rogeria exsulans). Nests of several species (belti, Rogeria blanda, merenbergiana) have been found under the loose bark of rotten logs. Nests of blanda and Rogeria tonduzi have been taken from the trunks of cacao trees. A nest of Rogeria leptonana was found at 1750m under a rock in a pasture.
Nests are rarely found. Males are known for only four species (belti, blanda, leptonana and Rogeria stigmatica) and queens associated through nest series for only nine species.
Castes
Queens have been collected but have not been described. Males have not been collected.
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- tonduzi. Rogeria tonduzi Forel, 1899c: 53 (w.) COSTA RICA. Combination in R. (Irogera): Emery, 1915i: 191; in Irogera: Brown, 1953h: 4 (in text); in Rogeria: Kempf, 1965: 185. See also: Kugler, C. 1994: 41.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Kugler (1994) - TL 3.0-3.2, HL 0.72-0.78, HW 0.61-0.68, SL 0.48-0.53, EL 0.12-0.15 (39-48 facets), PW 0.46-0.50, WL 0.81-0.90, SpL 0.18-0.21, PetL 0.37-0.40, PpetL 0.17-0.19mm, CI 0.85-0.88, OI 0.19-0.24, SI 0.78-0.81, PSI 0.20-0.25. N=7
Mandibles triangular; most specimens with 6 teeth, the first 5 decreasing in size basad then a large basal tooth. In others, the penultimate basal is replaced by 2-3 denticles. Clypeal apron convex with median angle or small tooth. Body of clypeus rises perpendicularly. Eyes oval with narrow anterior point. Nuchal groove inconspicuous.
Pronotum in lateral view lacks a distinct angle between anterior and dorsal faces. Metanotal groove very weak to absent. Propodeal spines long, weakly inclined. Postpetiole sub trapezoidal from above; sternum flat, with a distinct peduncle. Sting apparatus like that of inermis except for more elongate anterolateral processes on sting bulb.
Middorsum of head longitudinally rugose; laterodorsa and dorsal part of sides rugose-areolate. Mesosoma with thicker rugae and narrower interrugal spaces than on head. Rugae transverse on anterior pronotum, transverse to confused on metanotum and dorsal face of propodeum, predominantly longitudinal on sides and pronotal disc. Petiolar node longitudinally rugose on sides; smooth along midline. Postpetiole smooth. Microsculpture weak or absent throughout, integument very shiny.
Scapes and head with erect to suberect hair along with the typical short decumbent pilosity. Mesosoma dorsum and waist generally with erect to suberect hair of a variety of lengths; no decumbent-appressed pilosity. Gaster with long, erect hair and a few short, decumbent hairs.
Color shiny black with dark brown mandibles, scapes and legs to reddish-brown with yellowish-brown appendages.
Type Material
Kugler (1994) - Holotype worker, COSTA RICA (Tonduz) [MHN] [Holotype examined].
References
- Brown, W. L., Jr. 1953h. Characters and synonymies among the genera of ants. Part II. Breviora 18: 1-8 (page 4, Combination in Irogera)
- Emery, C. 1915g. Noms de sous-genres et de genres proposés pour la sous-famille des Myrmicinae. Modifications à la classification de ce groupe (Hymenoptera Formicidae). Bull. Soc. Entomol. Fr. 1915: 189-192 (page 191, Combination in R. (Irogera))
- Forel, A. 1899d. Formicidae. [part]. Biol. Cent.-Am. Hym. 3: 25-56 (page 53, worker described)
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- Kempf, W. W. 1965. Nota preliminar sôbre algumas formigas neotrópicas, descritas por Frederick Smith (Hymenoptera, Formicidae). Rev. Bras. Biol. 25: 181-186 (page 185, Combination in Rogeria)
- Kugler, C. 1994. A revision of the ant genus Rogeria with description of the sting apparatus (Hymenoptera: Formicidae). J. Hym. Res. 3: 17-89 (page 41, see also)
References based on Global Ant Biodiversity Informatics
- Ahuatzin D. A., E. J. Corro, A. Aguirre Jaimes, J. E. Valenzuela Gonzalez, R. Machado Feitosa, M. Cezar Ribeiro, J. Carlos Lopez Acosta, R. Coates, W. Dattilo. 2019. Forest cover drives leaf litter ant diversity in primary rainforest remnants within human-modified tropical landscapes. Biodiversity and Conservation 28(5): 1091-1107.
- Branstetter M. G. and L. Sáenz. 2012. Las hormigas (Hymenoptera: Formicidae) de Guatemala. Pp. 221-268 in: Cano E. B. and J. C. Schuster. (eds.) 2012. Biodiversidad de Guatemala. Volumen 2. Guatemala: Universidad del Valle de Guatemala, iv + 328 pp
- Dattilo W. et al. 2019. MEXICO ANTS: incidence and abundance along the Nearctic-Neotropical interface. Ecology https://doi.org/10.1002/ecy.2944
- De La Mora, A., and S. M. Philpott. 2010. Wood-nesting ants and their parasites in forests and coffee agroecosystems. Environmental Entomology 39: 1473-1481.
- De la Mora, A., C. J. Murnen, and S. M. Philpott. 2013. Local and landscape drivers of ant-communities in Neotropical coffee landscapes. Biodiversity and Conservation 22: 871-888.
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Franco W., N. Ladino, J. H. C. Delabie, A. Dejean, J. Orivel, M. Fichaux, S. Groc, M. Leponce, and R. M. Feitosa. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5): 509-543.
- Groc S., J. H. C. Delabie, F. Fernandez, F. Petitclerc, B. Corbara, M. Leponce, R. Cereghino, and A. Dejean. 2017. Litter-dwelling ants as bioindicators to gauge the sustainability of small arboreal monocultures embedded in the Amazonian rainforest. Ecological Indicators 82: 43-49.
- Groc S., J. H. C. Delabie, F. Fernandez, M. Leponce, J. Orivel, R. Silvestre, Heraldo L. Vasconcelos, and A. Dejean. 2013. Leaf-litter ant communities (Hymenoptera: Formicidae) in a pristine Guianese rainforest: stable functional structure versus high species turnover. Myrmecological News 19: 43-51.
- INBio Collection (via Gbif)
- Kempf W. W. 1961. Remarks on the ant genus Irogera Emery, with the description of a new species (Hymenoptera, Formicidae). Revista Brasileira de Biologia 21: 435-441.
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Kugler C. 1994. A revision of the ant genus Rogeria with description of the sting apparatus (Hymenoptera: Formicidae). Journal of Hymenoptera Research 3: 17-89.
- Longino J. T. 2013. Ants of Honduras. Consulted on 18 Jan 2013. https://sites.google.com/site/longinollama/reports/ants-of-honduras
- Longino J. T. L., and M. G. Branstetter. 2018. The truncated bell: an enigmatic but pervasive elevational diversity pattern in Middle American ants. Ecography 41: 1-12.
- Longino J. T., J. Coddington, and R. K. Colwell. 2002. The ant fauna of a tropical rain forest: estimating species richness three different ways. Ecology 83: 689-702.
- Longino J. T., and R. K. Colwell. 2011. Density compensation, species composition, and richness of ants on a neotropical elevational gradient. Ecosphere 2(3): 16pp.
- Longino J. et al. ADMAC project. Accessed on March 24th 2017 at https://sites.google.com/site/admacsite/

