Colobopsis markli
| Colobopsis markli | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Formicinae |
| Tribe: | Camponotini |
| Genus: | Colobopsis |
| Species: | C. markli |
| Binomial name | |
| Colobopsis markli Dumpert, 2004 | |
A monogynous species that nests in the plant Macanga griffithiana.
Identification
Maschwitz et al. (2004) - Major workers Distinctly larger than minor workers with respect to TL and the size of the head; no intermediates; distinctly phragmotic; all body parts – including the phragmotic part – are shining and not punctated; blackish to black in color, except the phragmotic part and the legs. Minor workers No intermediate forms between major and minor caste, head trapezoidal and elongated, all body parts are blackish and shining and not punctated. Decumbent pubescence is lacking, longer erect white hairs on all body parts. Queens Head elongated, sides are nearly straight. Eyes are situated behind the midlength of the sides of the head; ocelli widely spaced. Mesonotum prominent with steep declivities to pronotum and propodeum. Node of petiole flat on top. Color of head and gaster brown, alitrunk yellowish brown, legs darker. Males Head elongated, sides are nearly straight. Ocelli widely spaced. Color mostly mid brown, alitrunk and parts of the legs yellowish brown. Decumbent pubescence especially on gaster but also on the rest of the body parts.
We conclude that the ant partner of Macaranga griffithiana belongs to the Colobopsis group vitrea. Common characteristics: no intermediate forms between soldier and worker caste, phragmotic parts of the soldier and queens heads are not marginated, all anterior parts of the bodies of all castes are not grossly punctated.
Hitherto known species of Colobopsis (vitrea) are: Colobopsis gasseri from Australia, Colobopsis hosei from Borneo, and Colobopsis vitrea from Malaysia.
According to the original description by Forel – who only described workers and no soldiers and sexuals of this species – and syntypes from the Forel collection, C. hosei workers differ from those of C. markli in head shape, shape of the tibiae, pilosity and color. The head of C. hosei is as long as wide and the sides of the head are convex. In C. markli the heads are longer than wide and the sides are straight. The scapal length of C. hosei is distinctly shorter than in C. markli. The tibiae of C. hosei are cylindrical, in C. markli not. Pilosity of C. hosei decumbent, extremely short and yellowish. This contrasts to the pilosity of C. markli that is long, erect and white. Color of C. hosei is dark brown, excluding antennae and legs; in C. markli the whole body – including legs and antennae – is blackish brown.
Concerning C. gasseri we were also able to compare C. markli with the original description by Forel and syntypes of the Forel collection. Females of C. gasseri differ from C. markli females in several characteristics: occipital margin in full-face view straight, ocelli extremely small, petiolar scale in dorsal view rounded, color black except two broad yellow stripes on gaster; head not shining, rest of the body much more opaque than shining. Unlike C. markli, C. gasseri soldiers and workers are opaque on whole body surface; head of the C. gasseri soldiers distinctly striated especially between the frontal carinae.
The original description of C. vitrea was based on the worker caste only. In this species, the original description by Smith (1861) and determined specimens from the Forel collection were at our disposal. Different from C. markli are the following characteristics: occipital margin straight, head not elongated but as long as wide, whole animals are much longer and broader than workers of C. markli.
Besides material of C. vitrea we were also able to examine determined specimens of the subpecies C. vitrea angustatus Mayr from the Forel collection. This subspecies from Indonesia is distinctly smaller than C. vitrea. But also in this case, occipital margin is straight, head not elongated and pronotum distinctly broader than in C. markli. Besides, the color of C. vitrea angustatus is brown – instead of blackish brown as in the case of C. markli.
Distribution
Distribution based on Regional Taxon Lists
Oriental Region: Thailand (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
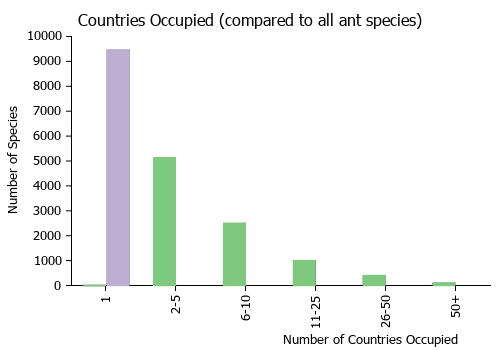
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
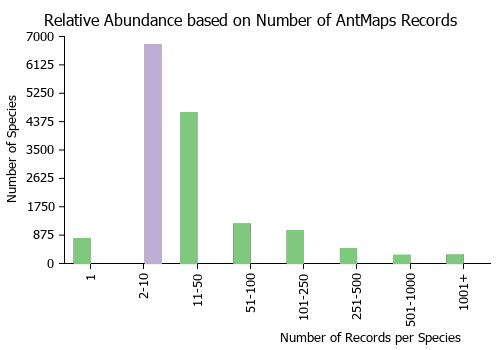
|
Biology
Maschwitz et al. (2004):
Whereas the southern population of M. griffithiana in the perhumid zone of the Malay Peninsula was inhabited by Crematogaster (Decacrema) msp.1, a disjunct northern population was associated with the black dimorphic Colobopsis markli. In three different separate subpopulations altogether 87 trees and treelets, including resprouts from cuttings, were checked in more detail. Of 29 young plants (0.5 to 2 m) only 21% were associated with Colobopsis markli although they already possessed swollen hollow stems. Of 58 larger plants (2 m to > 20 m), 81% were inhabited by C. markli. We found other ants only in 3 trees, one with an unidentified arboreal Crematogaster sp. (non-Decacrema) and two with a different Colobopsis species than the apparently Macaranga-specific C. markli (one of them together with a small colony of C. markli). Colobopsis markli could also be observed in two other small subpopulations of M. griffithiana. On more than 120 trees other than M. griffithiana checked in the close vicinity of the collection sites C. markli was not found.
Coccids. In all three populations most colonies were found to be associated with one coccid species in varying numbers, which could be identified as Coccus morphospecies 214 (Heckroth et al. 1998). One small subpopulation with about 10 C. markli colonies contained no coccids. Nevertheless, colonies here were also well developed and contained brood. The coccid species was already known from various Macaranga-ant associations, also from M. motleyana, the sister species of M. griffithiana in Borneo. It is a rather polyphagous endophytic scale insect which was, in contrast to most coccids collected from Macaranga, also rarely (n = 7) found in other hollow plants, such as Myrmeconauclea, and even in non-myrmecophytic lianas like Uncaria, and was tended by unspecific arboreal Crematogaster ants (Heckroth et al. 1998).
Colony structure, ant behavior and plant-ant characters
During our survey of M. griffithiana saplings we never found a Colobopsis markli colony with more than one queen, possibly indicating monogyny in this species. We did not, however, dissect whole trees with large colonies. The nodal regions of the stems and branches were mostly hollowed out by the ants. Thus a widely uninterrupted passage through the plant resulted. The round entrance holes in the internodes looked very regular, were obviously bitten by the ants and had a diameter of 1–2.5 mm (average x– = 1.69 ± 0.31 SD, n = 15). They were partly closed by callus growth in the lower parts of the tree.
Similar to the Colobopsis sp. association with M. puncticulata (Federle et al. 1998a, b), larger colonies often apparently occupied several trees. This was indicated by the fact that no fertile queens could be found in smaller trees close to larger ones with a physogastric queen. One such cluster consisting of seven trees between 3.5 and 6.5 m in height, growing in a rectangle of 2.5m2, was thoroughly checked for its ant inhabitants in more detail: only in one central tree, 5.5 m in height, did we find a strongly physogastric queen in the stem interior about 3 m up. All trees contained ant brood (including the cocoonless pupae), adult ant workers, soldiers, many alate young queens, and in one tree two males.
The workers were observed to patrol on the tree and collect food bodies. In larger colonies the workers attacked human disturbers by biting, but not in a very aggressive way. Though we have not observed pruning behavior directly, C. markli very likely shows this behavior. As typical for pruning plantants, well colonized trees were free from vines as a rule, whereas uncolonized trees often were more or less covered by vines. In four well accessible trees at the forest edge we observed freshly destroyed (with biting marks) shoots of Mikania sp. (Asteraceae), a fast growing non-woody climber, which occurred at such sites on well exposed bushes and trees in the surroundings. This climber was also abundant nearby on bushy M. griffithiana without ant inhabitants.
Colony foundation of C. markli
We discovered only five colony founding-queens in saplings of between 1.8 and 2.5 m in height. These foundation colonies, however, looked very similar to those of other typical specialized plant ants in the Macaranga system. Foundation appeared to occur claustrally, with the queen biting an entrance hole into an internode which afterwards closed. Two young queens were found with brood but were still workerless. The round entrance holes of the still claustral queens had begun to close by growth but still contained remains of a carton closure material. Three queens were with a few small workers which had bitten a small hole near an old larger entrance hole, apparently formerly bitten by the queen and subsequently closed by plant growth. All five foundation chambers contained no scale insects, indicating that the young scales likely arrived as crawlers by wind transport as in the Macaranga–Crematogaster associations (Heckroth et al. 1998) and that the large queens raise their first offspring using their own body reserves without the help of any trophobionts.
Queens of C. markli were found in the upper third of the trees. This is as in Colobopsis macarangae, but in contrast to primary colony foundations of Crematogaster (Decacrema) in other Macaranga species where the first colony-founding queens remain in lower parts of the stem. However, we have also found colonizing queens of Crematogaster (Decacrema) msp. 1 in Macaranga griffithiana in higher stem parts than in other Macaranga species (as high as 1.5 m) perhaps due to the fact that the stem diameter often remains rather small up to a sapling size of about 0.5–0.7 m. In addition, the plants grow in swamps and lower stem parts might become subject to flooding in the rainy season. One can also not rule out the possibility that we have to deal with a succession of different colonizing queens during the ontogeny of the plant. We found taller trees of M. griffithiana in Peninsula Malaysia that were newly colonized by Decacrema msp. 1 queens which were then located in the branches of the tree and not in the trunk (Feldhaar et al. 2003a). Such a colonization of the crown region might also occur with Colobopsis markli.
The degree of ant-colonization was locally rather low, which was also the case in C. (Colobopsis)- M. puncticulata associations. At two sites we found destroyed or partly destroyed colonies, the domatia of which were torn open in a similar way as observed in various Macaranga species in Peninsular Malaysia and in Borneo. There, mainly squirrels were identified as the destroyers (Federle et al. 1999) but also birds were observed (B. Fiala, pers. observation). A further reason, however, might be the very disturbed and fragmented habitat. Extensive open areas might limit the dispersal of the colonizing queens (Fiala et al. 1999).
Associated Species
Several characters of the association partners are characteristic of close myrmecophytic relationships:
– maintenance of the full set of ant-plant characters in M. griffithiana, i.e., formation of thin-walled, fragile domatia, and a high rate of food body production,
– the high specificity of associations at several separated subpopulations in open and in forested habitats, monopolization of trees by colonizing ants although other arboreal ant species were present,
– host plant localization, and domatium opening behavior (chewing of entrance holes into living plant tissue) of the colony-founding queen,
– frequent food body consumption by the partner ant,
– aggressiveness and probable pruning behavior (this was also reported from Colobopsis colonizing M. puncticulata, Federle et al. 1998a),
– presence of a single coccid partner species. This homopteran tending as well as food body harvesting can make the ants independent of food searching away from the plant.
All these characters taken together speak for a close association of all three partners. It obviously represents another example of the independent origin of a myrmecophytic association within the diverse genus Macaranga. The two other associations of Macaranga plants with Colobopsis species differ in many aspects to the one reported in this study. The Macaranga species involved are not closely related, M. griffithiana (West Malaysia, Thailand) and Macaranga lamellata (Borneo) belong to different clades within the section Pachystemon, and Macaranga puncticulata (West Malaysia) is even more distant, forming an own clade (Blattner et al. 2001, Davies 2001). On the ant side as well, initial phylogenetic analyses based on mitochondrial DNA sequence data of all Camponotus species involved revealed that C. markli is not closely related to either of the other Macaranga-colonizing Colobopsis species (J. Gadau et al., unpubl. results), i.e., has obviously evolved independently as a plant-ant.
Castes
Worker
Images from AntWeb
   
| |
| Paratype of Camponotus markli. Worker. Specimen code casent0911639. Photographer Z. Lieberman, uploaded by California Academy of Sciences. | Owned by NHMB, Basel, Switzerland. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- markli. Camponotus (Colopobsis) markli Dumpert, in Maschwitz, Fiala & Dumpert, 2004: 44, figs. 2-5 (s.w.q.m.) THAILAND.
- Type-material: holotype major worker, 6 paratype major workers, 7 paratype minor workers, 6 paratype queens, 2 paratype males.
- Type-locality: holotype Thailand: Khao Chamao, 1.ii.2001 (U. Maschwitz); paratypes: 4 major workers, 7 minor workers, 3 queens, 2 males with same data, 2 major workers, 3 queens Thailand: Khao Sa Bap, 3.ii.2001 (U. Maschwitz).
- Type-depositories: NHMB (holotype); MCZC, MNKL, NHMB, UMPC (paratypes).
- Combination in Colobopsis: Ward, Blaimer & Fisher, 2016: 350.
- Status as species: McArthur, 2012: 72; Khachonpisitsak, et al. 2020: 49.
- Distribution: Thailand.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Holotype: TL 4.1, HW 0.95, HL 0.98, CI 96.9, OD 0.27, SL 0.66, SI 69.5, PW 0.62, PH 0.38, PB 0.24.
Head nearly as wide as long (CI 96.9), sides are nearly straight and not divergent. Eyes are situated behind the midlength of the sides of the head and slightly protruding; their maximum diameter is 0.27 mm or 0.28 HW. Occipital margin and corners in full-face view strongly rounded (Fig. 2 a). Antennal insertion right in the midlength of frontal carinae. Clypeus not convex but strongly angled downwards, forming – together with a small part of the genae and the mandibles – the phragmotic part of the head (Fig. 2 b). Anterior clypeal margin rounded. Mandibles much broader than those of the minors, with lateral borders strongly curved and 5 strong teeth on each masticatory border (Fig. 2 c). Antennal scapes relatively short (SI 69.5); they just reach the occipital margin. Funicular segments become continuously smaller and do not form a distinct club. Frontal carinae extend to less than two-thirds of head length. Apart from a slight projection behind the scapal insertion, they are straight and distinctly divergent. Alitrunk with a deep impression (metanotum) between promesonotum and propodeum, and two raised stigmata beside the deepest point of the impression. Petiolar scale broadly rounded on top.
Nearly whole animal blackish brown to black in color; only legs and front part of head lighter. Surface of all body parts shining. Accordingly, cuticular punctures and reticulated sculptures (SEM) are weak (Fig. 2 a). Decumbent pubescence is lacking on all body parts. Longer erect white hairs on all body parts, especially on antennal scapes and legs.
Paratype (majors) (n = 6): TL 4.1–4.5, HW 0.94–1.06, HL 0.98–1.30, CI 86.1–96.9, OD 0.27–0.3, SL 0.65–0.74, SI 63.7–70.2, PW 0.6–0.68, PH 0.38–0.46, PB 0.23–0.28.
Head trapezoidal and elongated (CI 83.7), sides are nearly straight. Eyes are situated behind the midlength of the sides of the head and slightly protruding; their maximum diameter is 0.23 mm or 0.32 HW. Occipital margin and corners in full face view strongly rounded (Fig. 3 a). Clypeus slightly convex and without median carina; anterior clypeal margin rounded. Mandibles relatively short, with lateral borders strongly curved and 4 subequal teeth on each masticatory border. Antennal scapes relatively large (SI 97.2); they project beyond the occipital margin by about one third of their length. Funicular segments become continuously smaller and do not form a distinct club. Frontal carinae extend to about two-thirds of head length. Apart from a slight projection behind the scapal insertion, they are straight and slightly divergent (Fig. 3 a). Alitrunk and petiolar scale as in major worker.
Whole animal dark brown to black in color, only tarsi and mandibles lighter. Surface of all body parts shining. Accordingly, cuticular punctures and reticulated sculptures (SEM) are weak (Figs. 3 a, b). Decumbent pubescence is lacking on all body parts. Longer erect white hairs on all body parts, especially on antennal scapes and legs.
Paratype (minors) (n = 7): TL 3.1–3.4, HW 0.72–0.78, HL 0.86–0.94, CI 85.2–88.3, OD 0.23–0.24, SL 0.68–0.74, SI 92.3–97.3, PW 0.44–0.5, PH 0.34–0.36, PB 0.2–0.22.
Queen
Head elongated (CI 73.3–74.6), sides are nearly straight. Eyes are situated behind the midlength of the sides of the head and distinctly protruding; their maximum diameter is 0.50–0,52 mm or 0.45–0.48 HW. Ocelli widely spaced (OD1 0.28–0.34 mm, OD2 0.16–0.18 mm). Occipital margin and corners in full-face view strongly rounded (Fig. 4 a). Clypeus not convex but strongly angled downwards, forming – together with a small part of the genae and the mandibles – the phragmotic part of the head (Fig. 4 b). Anterior clypeal margin rounded. Mandibles relatively short, with lateral borders strongly curved and 6 subequal teeth on each masticatory border. Position of antennal insertion right in the midlength of frontal carinae. Antennal scapes relatively short (SI 80.4–97.6); they are long enough to reach the occipital margin. Funicular segments become continuously smaller and do not form a distinct club. Frontal carinae extend to nearly two-thirds of head length. Apart from a slight projection behind the scapal insertion, they are straight and slightly divergent (Fig. 4 a). Alitrunk in dorsal view with the pronotal angles weakly rounded. Seen in profile, mesonotum prominent with steep declivities to pronotum and propodeum, surface of mesonotum only slightly rounded (Fig. 4 c). Petiolar scale in dorsal view broadest behind with rounded corners; seen in profile, node of petiole flat on top, anterior slope steep, posterior slope broadly rounded on top (Fig. 4 d).
Color of head and gaster brown, alitrunk yellowish brown; legs darker. Surface of all body parts shining. Accordingly, cuticular punctures and reticulated sculptures (SEM) are weak (Figs. 4 a, b, c). Decumbent pubescence scarce on all body parts. Longer erect white hairs on all body parts, especially on antennal scapes and legs.
Paratype (females) (n = 6): TL 7.6–7.9, HW 1.08–1.12, HL 1.46–1.52, CI 73.3–74.6, OD 0.50–0.52, OD1 0.28–0.34, OD2 0.16–0.18, SL 0.86–0.96, SI 80.4–97.6, PW 1.15–1.24, PH 0.44–0.46, PB 0.42–0.48, FWL 7.5–7.8, HWL 5.0–5.1, FWW 2.2–2.2, HWW 1.7–1.7.
Male
Head elongated (CI 76.9–89.2), sides are nearly straight (fig.5 a). Eyes are situated midlength of the sides of the head and strongly protruding; their maximum diameter is 0.31–0.33 mm or 0.46–0.55 HW. Ocelli widely spaced (OD1 0.22–0.24 mm, OD2 0.10–0,12 mm). Occipital margin and corners in full-face view strongly rounded (Fig. 5 b). Clypeus strongly convex and with distinct median carina; anterior clypeal margin strongly rounded. Mandibles relatively short, with lateral borders scarcely curved and 3 subequal teeth on each masticatory border which are more or less rounded (Fig. 5 b). Antennal scapes moderately long (SI 72.7 – 83.3); they are just long enough to reach the occipital margin (Fig. 5 a). Funicular segments about equal in width. Frontal carinae extend to about midlength of the head. Apart from a slight projection behind the scapal insertion, they are straight and distinctly divergent (Fig. 5 b). Alitrunk with only a very slight impression between promesonotum and propodeum (Fig. 5 c). Petiolar scale stongly rounded on top.
Color mostly mid brown, altirunk and parts of the legs yellowish brown. Surface of all body parts shining. Accordingly, cuticular punctures and reticulated sculptures (SEM) are weak (Figs. 5 a, b). Decumbent pubescence especially on gaster but also on the rest of the body parts. Longer erect white hairs on all body parts, especially on antennal scapes and legs.
Paratype (males): TL 3.82–3.83, HW 0.60–0.66, HL 0.74–0.78, CI 76.9–89.2, OD 0.31–0.33, OD1 0.22–0.24, OD2 0.10–0.12, SL 0.48–0.5, SI 72.7–83.3, FWL 4.6–4.7, HWL 2.9–3.0, FWW 1.7–1.7, HWW 1.3–1.3.
Type Material
Holotype, eastern Thailand: Khao Chamao, 1.02.2001, leg. U. Maschwitz (Naturhistorisches Museum Basel).
Paratype (majors) 4 with the same data as holotype (2 in Museum of Comparative Zoology at Harvard University, 2 in National Museum of Malaysia, Kuala Lumpur) and 2 eastern Thailand: Khao Sa Bap, 3.02.2001, leg. U. Maschwitz (in collection of the author).
Paratype (minors): 7 from eastern Thailand: Khao Chamao, 1. 02. 2001, leg. U. Maschwitz (1 in Naturhistorisches Museum Basel, 2 in Museum of Comparative Zoology at Harvard University, 2 in National Museum of Malaysia, Kuala Lumpur, 3 in collection of the author).
Paratype (females): 3 from eastern Thailand: Khao Chamao, 1.02.2001, leg. U. Maschwitz (1 in Naturhistorisches Museum Basel, 1 in Museum of Comparative Zoology at Harvard University, 1 in National Museum of Malaysia, Kuala Lumpur), 3 eastern Thailand: Khao Sa Bap, 3.02.2001, leg U. Maschwitz (in collection of the author).
Paratype (males), 2 from eastern Thailand: Khao Chamao, 1. 02. 2001, leg. U. Maschwitz (1 Naturhistorisches Museum Basel, 1 collection of the author).
Etymology
The species name is dedicated to the great biologist, myrmecologist and president of the Max-Planck-Gesellschaft Prof. Dr. Hubert Markl.
References
- Dumpert, K. 2004. Description of the ant species. Pp. 41-48 in: Maschwitz, U.; Fiala, B.; Dumpert, K. 2004. An unusual myrmecophytic Macaranga association, occurring in a disjunct area in the monsoon zone of south-east Asia: phenology and the description (page 44, worker described)
- Khachonpisitsak, S., Yamane, S., Sriwichai, P., Jaitrong, W. 2020. An updated checklist of the ants of Thailand (Hymenoptera, Formicidae). ZooKeys 998, 1–182 (doi:10.3897/zookeys.998.54902).
- Ward, P.S., Blaimer, B.B., Fisher, B.L. 2016. A revised phylogenetic classification of the ant subfamily Formicinae (Hymenoptera: Formicidae), with resurrection of the genera Colobopsis and Dinomyrmex. Zootaxa 4072 (3): 343–357 (doi:10.11646/zootaxa.4072.3.4).
References based on Global Ant Biodiversity Informatics
- Maschwitz U., B. Fiala, and K. Dumpert. 2004. An unusual myrmecophytic Macaranga association, occurring in a disjunct area in the monsoon zone of south-east Asia: phenology and the description of a new ant species. Ecotropica 10: 33-49.

