Eurhopalothrix floridana
| Eurhopalothrix floridana | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Eurhopalothrix |
| Species: | E. floridana |
| Binomial name | |
| Eurhopalothrix floridana Brown & Kempf, 1960 | |
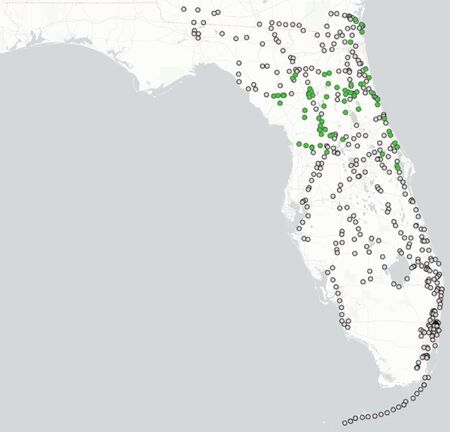
Longino (2013): Deyrup et al. (1997) reviewed the status of E. floridana in Florida and described the male. The species was moderately abundant in hardwood hammocks throughout peninsular Florida but did not extend into the western panhandle. They speculated that the species could be exotic in Florida, and referred to a personal communication from W. L. Brown that the species occurred in Mexico. I have seen no material to verify the occurrence in Mexico, but J. Wetterer collected specimens on Mona Island, Puerto Rico, and D. Lubertazzi collected specimens along a roadside in the Dominican Republic. The biogeographic history of this species, in Florida and elsewhere, remains enigmatic.
Identification
Longino (2013): The specimens from Mona Island, Puerto Rico and the Dominican Republic are nearly identical to specimens from Florida, although there is some variation in number of erect setae. On the two Mona Island specimens, one has a cluster of 2 setae on the face and one has 3. Both have the full complement of 6 setae on the promesonotum. On the two Dominican Republic specimens, one has a cluster of 3 setae on the face, 3 setae down one side of the promesonotum, and 1 seta on the opposite side, paired with the posteriormost. The other one has no setae on the face and 2 on the mesonotum. How much these patterns are due to seta loss is unknown, but the base number could be a cluster of 4 on the face, like Eurhopalothrix apharogonia, and 6 on the promesonotum.
Keys including this Species
Distribution
Deyrup et al. (1997): Brown and Kempf (1960) suggest that E. floridana could be a recent introduction from the Neotropics. There is additional circumstantial evidence that supports this suggestion. Expanding ranges are typical of exotic species; E. floridana is now easily obtained in Alachua and Putnam counties, where Van Pelt (1958) sampled extensively for dacetine ants without finding E. floridana. Eurhopalothrix floridana has not been found in the West Indies (changed since this was stated here in 1998, see Longino 2013), the source of almost all tropical ants that have, we assume, dispersed naturally to Florida. It is unlikely to be a native from the forests of the southern Appalachians, because such species usually have a large range to the north and west of Florida. E. floridana occurs in Mexico (W. L. Brown, Jr., 1985, pers. comm.), so there is a possible source of introduction. There are species of insects, including ants, that occur in southwestern North America, with a disjunct population or closely related congeners in Florida, but these are species of open, desert or savannah habitats, not woodlands (Deyrup 1990).
On the other hand, it could be native to Florida. The oldest record is a damaged specimen found recently by David Smith of the U. S. National Museum of Natural History. This specimen is from Key West (once a major commercial port), dated 1887, and is in the Pergande collection. A record of this antiquity coming from a less settled part of Florida might suggest that E. floridana is a native species, but in tropical Florida a great amount of entrepreneurial horticulture occurred well before 1887. Henry Perrine, for example, was in a good position in the 1830's to be an unintentional purveyor of cryptobiotic ants to the Miami area. Perrine displayed a missionary zeal in the importation of plants from southern Mexico, many in boxes or tubs of soil. Marjory Douglas (1978) cites a report of "more than 100 boxes of plants shipped from the Yucatan" by Perrine outside one building in the Miami area. Eurhopalothrix floridana is a cryptic species that could have escaped being, noticed by some collectors; it does not show any association with highly disturbed habitats. Its ability to live in xeric woodlands might have allowed it to move to Florida from Mexico along with other upland species at the end of the Pliocene or in the early Pleistocene. We conclude that this species is probably introduced into Florida, but would like to see better documentation of the species in Mexico, especially southern Mexico.
Latitudinal Distribution Pattern
Latitudinal Range: 30.73333333° to 17.928702°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Nearctic Region: United States (type locality).
Neotropical Region: Dominican Republic, Puerto Rico.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
Deyrup et al. (1997) - Eurhopalothrix floridana occurs throughout peninsular Florida, based on about 130 collections from over 70 sites. Although we sampled leaf litter and rotten wood from all over Florida, we made no effort to sample evenly, and the distribution pattern reflects biases based on convenience and access. The total sampling effort, however, involved many thousands of Tulgren funnel extractions, collected in all regions of the stale. We collected, for example, hundreds of samples from the western panhandle, without finding any E. floridana. The pattern of records reflects the distribution of woodlands in peninsular Florida, including the dry tropical hammocks of the Florida Keys, the Atlantic coastal ridge hammocks, the scrub forests and ecotonal hardwood forests of the southern and central ridges, and the mesic and xeric forests of the northern peninsula.
In a study of E. floridana using standardized, unsifted, approximately 2-liter samples of litter, we found E. floridana in 65 samples on 32 occasions. The 32 habitat records include: 8 from xeric forest (old growth sand pine scrub, sandhill invaded by large oaks), 11 from mesic forest (usually mixed oaks and pines), 5 from wet forest (mixed pine and hardwoods, usually induding oaks and magnolia); and 8 from coastal tropical hardwood forest. This is apparently a woodland species that is not particular about drainage.
The 2-liter litter samples used in this analysis were indexed by site and date, with an ant species list for each sample. This allows us to say that, out of 346 samples collected at a site and date where E. floridana was found, 65 (19%) contained an E. floridana. At a number of sites less than one out of 10 samples had E. floridana, so it is evident that in many cases at least 5, and often more, samples must be extracted to demonstrate the presence on this species at a particular site. This need for large numbers of samples to show presence or absence of a species is a common problem in surveys of litter ants; many species are much rarer.
In our small litter samples, E. floridana is usually found with other species of ants, with which it must be somewhat compatible and with which it must share microhabitat requirements. The numbers of co-occurring species are as follows: 6 samples had no other ant species, 12 samples had l other species; 15 samples had 2 other species; 13 samples had 3 other species, 11 samples had 4 other species, 6 samples had 5 other species, and 2 samples had 6 other species.
Seasonality of males: males were collected with workers on 12 July and 19 August. Fifty males were collected in Townes traps at several sites between 15 July and 5 December.
Atchison & Lucky (2022) found that this species does not remove seeds.
Castes
Worker
Images from AntWeb
   
| |
| Worker. Specimen code casent0006100. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Queen
Images from AntWeb
   
| |
| Queen (alate/dealate). Specimen code casent0104864. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
    
| |
| Queen (alate/dealate). Specimen code casent0103902. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
Male
Images from AntWeb
    
| |
| Male (alate). Specimen code casent0103905. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- floridana. Eurhopalothrix floridana Brown & Kempf, 1960: 207, fig. 34 (w.) U.S.A. Deyrup, Johnson & Davis, 1997: 183 (m.).
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Longino (2013) - HW 0.62–0.71, HL 0.61–0.70, SL 0.40–0.45, SLL 0.08–0.10, CI 95–102, SLI 20–25 (n=4). Labrum longer than broad, anterolateral gibbosities of basal portion developed as acute, ventrally-directed teeth, apical portion bluntly rounded apically, not at all bilobed; apex of labrum with fringe of thick translucent setae; mandible triangular, dorsal surface convex, punctulate, rounding into ventral surface; interior surface concave, smooth and shining; masticatory margin with two tooth rows, an outer row of 10 teeth and an inner row of 3 long needle-shaped teeth, behind outer teeth 3–6 and projecting beyond them, nearly 2x length of flanking outer teeth; tooth 1 of outer row broader than others at base; tooth 2 long and acute; teeth 3–6 low, blunt; teeth 7 and 10 long and acute; teeth 8–9 shorter; scape with strongly developed basal lobe; scrobe deep, sharply delimited dorsally and ventrally, abutting deep antennal socket; surface of scrobe foveolate; eye with about 5 ommatidia across greatest diameter; clypeus convex posteromedially, sloping to slightly concave anterior apron, roughened; juncture of clypeus and frons impressed; sides of head above eyes strongly angulate; surface of face uniformly convex, roughened to shallowly rugulose; occipital carina a short carina dorsally, obsolete laterally; undersurface of head shallowly rugulose; postgenal suture a well-developed longitudinal trough.
Profile of promesonotum and dorsal face of propodeum forming more or less continuous convexity; promesonotal suture very faintly impressed on some specimens, such that mesonotum slightly differentiated from pronotum; metanotal groove impressed; dorsal and posterior faces of propodeum distinct, meeting at obtuse angle, dorsal face subequal in length to posterior face; propodeal spine laminar, translucent, acute, ventral margin continuous with relatively broad infradental lamella that extends down posterior face to propodeal lobe, ventral border straight to slightly concave; propodeal spiracle distinct, directed posteriorly; most of mesosoma, including posterior face of propodeum, uniformly covered with small puncta; mesonotum also uniformly punctate or sometimes slightly roughened like face; metapleural gland bulla smooth, matte; lacking transverse carinulae between propodeal spines.
Petiolar peduncle joins anterior face of petiolar node at obtuse angle; petiolar node subquadrate, anterior face rounding into convex dorsal face, which rounds into short posterior face; ventral margin of petiole with acute anteroventral tooth; postpetiole low and broad, with a shallow longitudinal sulcus dorsally; first gastral sternite lacking anterior sagittal keel; petiole, postpetiole, first gastral tergite covered with dense, small, puncta, interspaces less than or equal to width of puncta; first gastral sternite similar, but puncta and interspaces larger.
Dorsal surface of scape with uniform, short, appressed, flattened setae; leading edge of scape with projecting setae, shortest near apex, gradually lengthening to longest on basal lobe; ground pilosity on face similar to that on dorsal scape, uniformly distributed across face, frontal lobes, and clypeus, those on clypeus longitudinally oriented; undersurface of head with ground setae like those on face; projecting specialized setae strongly clavate to somewhat spatulate, 3–4x longer than wide, full complement 2, on vertex margin; ground pilosity sparse on promesonotal dorsum, petiolar node, and first gastral tergite, denser on postpetiole; 3 pairs projecting clavate setae on promesonotum; legs with moderately abundant, flattened, appressed setae on apices of femora, posterior face of foretibia, entire midtibia, anterior face of hindtibia, somewhat sparser on other surfaces; apex of foretibia with 1 larger clavate seta, apices of mid and hind tibia with 2; basitarsus and remaining tarsomeres with abundant, clavate setae; two clavate setae on hind margin of dorsal face of petiolar node; row of 4 clavate setae on hind margin of postpetiole; specialized setae of first gastral tergite clavate, full complement 3 pairs in two longitudinal rows.
Color red brown.
Queen
Longino (2013) - Although the queen has never been formally reported, images of a queen on AntWeb (CASENT0103902, from Florida) show it to have the typical similarities to the worker.
Male
Deyrup et al. (1997) - The male generally resembles a male dacetine in its dense, reticulate sculpture, the facial projection from which the antennae emerge, reduced venation, and long petiole. It is distinguished from Florida dacetines by the following features: 1.) The post petiole is twice as wide as the petiole, with a strongly concave anterior border and a conspicuous spatulate hair on each side. 2.) The first submarginal cell is clearly developed. 3.) The antennal scape has a strong bristle on the proximal corner of the large bulge on the inner side. This may be a character found throughout the genus (Brown and Kempf 1960) 4.) The size is relatively large. Total length: 2.02-2.15 mm. Length of forewing: 2.32-2.45 mm.
Type Material
- Holotype, worker, Highlands Hammock, Highlands Co., Florida, United States, 15 Jun 1955, H. S. Dybas, H. S. Dybas, No. B-31, Field Museum of Natural History; in leaf litter.
References
- Atchison, R. A., Lucky, A. 2022. Diversity and resilience of seed-removing ant species in Longleaf Sandhill to frequent fire. Diversity 14, 1012 (doi:10.3390/d14121012).
- Brown, W. L., Jr.; Kempf, W. W. 1960. A world revision of the ant tribe Basicerotini. Stud. Entomol. (n.s.) 3: 161-250 (page 207, fig. 34 worker described)
- Chaul, J.C.M. 2022. Redescription of Eurhopalothrix reichenspergeri (Santschi, 1923) stat. rev. (Hymenoptera: Formicidae), a Brazilian Atlantic Forest endemic species. Zootaxa 51821, 1-20 (doi:10.11646/zootaxa.5182.1.1).
- Deyrup, M., Davis, L. & Cover, S. 2000. Exotic ants in Florida. Transactions of the American Entomological Society 126, 293-325.
- Deyrup, M.; Johnson, C.; Davis, L. 1997. Notes on the ant Eurhopalothrix floridana, with a description of the male (Hymenoptera: Formicidae). Entomol. News 108: 183-189 (page 183, male described)
- Deyrup, M.A., Carlin, N., Trager, J., Umphrey, G. 1988. A review of the ants of the Florida Keys. Florida Entomologist 71: 163-176.
- Longino J. T. 2013. A review of the Central American and Caribbean species of the ant genus Eurhopalothrix Brown and Kempf, 1961 (Hymenoptera, Formicidae), with a key to New World species. Zootaxa 3693: 101-151 (doi:10.11646/zootaxa.3693.2.1).
- Lubertazzi, D. 2019. The ants of Hispaniola. Bulletin of the Museum of Comparative Zoology, 162(2), 59-210 (doi:10.3099/mcz-43.1).
- Wetterer, J.K., Booher, D.B. 2023. Geographic distribution of Eurhopalothrix floridana (Hymenoptera: Formicidae). Transactions of the American Entomological Society, 149(3), 315-324 (doi:10.3157/061.149.0303).
References based on Global Ant Biodiversity Informatics
- Annotated Ant Species List Ordway-Swisher Biological Station. Downloaded at http://ordway-swisher.ufl.edu/species/os-hymenoptera.htm on 5th Oct 2010.
- Baroni Urbani C., and M. L. De Andrade. 2007. The ant tribe Dacetini: limits and constituent genera, with descriptions of new species (Hymenoptera, Formicidae).
. Annali del Museo Civico di Storia Naturale "Giacomo Doria" 99: 1-191. - Brown W. L., Jr., and W. W. Kempf. 1960. A world revision of the ant tribe Basicerotini. Stud. Entomol. (n.s.) 3: 161-250.
- Deyrup M., C. Johnson, G. C. Wheeler, J. Wheeler. 1989. A preliminary list of the ants of Florida. Florida Entomologist 72: 91-101
- Deyrup M., C. Johnson, and L. Davis. 1997. Notes on the ant Eurhopalothrix floridana, with a description of the male (Hymenoptera: Formicidae). Entomological News 108: 183-189.
- Deyrup, M. 2003. An updated list of Florida ants (Hymenoptera: Formicidae). Florida Entomologist 86(1):43-48.
- Deyrup, M. and J. Trager. 1986. Ants of the Archbold Biological Station, Highlands County, Florida (Hymenoptera: Formicidae). Florida Entomologist 69(1):206-228
- Johnson C. 1986. A north Florida ant fauna (Hymenoptera: Formicidae). Insecta Mundi 1: 243-246
- Longino J. T. 2013. A review of the Central American and Caribbean species of the ant genus Eurhopalothrix Brown and Kempf, 1961 (Hymenoptera, Formicidae), with a key to New World species. Zootaxa 3693(2): 101-151.
- Longino, J.T. 2010. Personal Communication. Longino Collection Database
- Moreau C. S., M. A. Deyrup, and L. R. David Jr. 2014. Ants of the Florida Keys: Species Accounts, Biogeography, and Conservation (Hymenoptera: Formicidae). J. Insect Sci. 14(295): DOI: 10.1093/jisesa/ieu157