Pseudoponera gilberti
| Pseudoponera gilberti | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Genus: | Pseudoponera |
| Species: | P. gilberti |
| Binomial name | |
| Pseudoponera gilberti (Kempf, 1960) | |
From Mackay and Mackay (2010): One colony was found in a well-rotted Nasutitermes nest on the forest floor, another in the soil under leaf litter. A colony was collected in rotten wood in Perú in a site with sandy soil. A specimen was extracted from a soil sample and a second worker was extracted from palm flower litter. A dealate female was collected in July (Ecuador) in a statry bivouac site of the army ant Eciton burchellii just after emigration. Other females were collected in February and December (Kempf, 1960a).
Identification
From Mackay and Mackay (2010): The worker and female of P. gilberti are essentially identical to those of Pseudoponera stigma, as both species have a broadly rounded subpetiolar lobe and six mandibular teeth. The major difference between them is that the transverse carina on the clypeus of P. gilberti is well developed, but only poorly developed or nearly absent in P. stigma. Pseudoponera gilberti is also similar to Pseudoponera succedanea, but can be distinguished as the posterior edge of the subpetiolar process is rounded, not angulate as in P. succedanea.
Separation of the males of P. gilberti from those of P. stigma is difficult. The smaller medium brown specimens can be separated from the larger dark brown males of P. stigma. The large dark males of P. gilberti appear to be indistinguishable from similar males of P. stigma.
Distribution
Southern Central America through central South America, Trinidad. (Mackay and Mackay 2010)
Latitudinal Distribution Pattern
Latitudinal Range: 8.78° to -22.468°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Brazil (type locality), Ecuador, Suriname, Venezuela.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
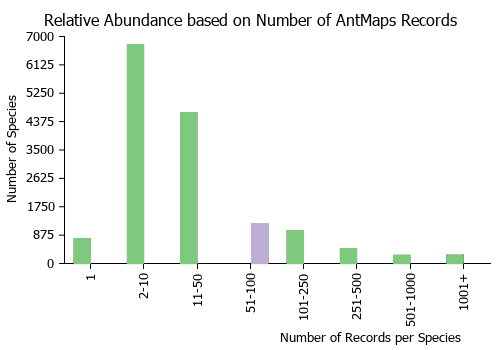
|
Habitat
Pseudoponera gilberti was found in the transition between tierra firme and seasonally flooded forest clearing in secondary forest, from 0 to 325 meters. (Mackay and Mackay 2010)
Biology
Castes
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- gilberti. Trachymesopus gilberti Kempf, 1960f: 425, fig. 6 (w.q.) BRAZIL (São Paulo, Rio de Janeiro).
- Type-material: holotype worker, 3 paratype queens.
- Type-locality: holotype Brazil: São Paulo, Agudos, 7.i.1959 (C. Gilbert); paratypes: 2 queens São Paulo, Agudos, 23.ii.1955, and 4.xii.1955 (W.W. Kempf), 1 queen Rio de Janeiro, Pôrto das Caixas, ii.1928 (O. Conde).
- Type-depository: MZSP.
- Wheeler, G.C. & Wheeler, J. 1971b: 1206 (l.); Mackay & Mackay, 2010: 344 (m.).
- Combination in Mesoponera: Wheeler, G.C. & Wheeler, J. 1971b: 1206;
- combination in Pachycondyla: Brown, in Bolton, 1995b: 305;
- combination in Pseudoponera: Schmidt, C.A. & Shattuck, 2014: 208.
- Status as species: Kempf, 1961b: 494; Kempf, 1972a: 251; Brandão, 1991: 356; Bolton, 1995b: 305; Mackay & Mackay, 2010: 343 (redescription); Bezděčková, et al. 2015: 125; Feitosa, 2015c: 99.
- Distribution: Brazil, Colombia, Ecuador, French Guiana, Guyana, Panama, Peru, Suriname, Trinidad.
Type Material
- Paratype, queen, Agudos, São Paulo, Brazil, Museum of Comparative Zoology; see Mackay and Mackay 2010.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
From Mackay and Mackay (2010): The worker is a small (total length 5 mm) dark reddish brown ant. The mandibles have 6 teeth. The transverse carina on the clypeus is well developed and a longitudinal medial area is swollen, but does not form a carina. The head is narrowed anteriorly and the posterior border is concave. The eye is small (maximum diameter 0.07 mm) located slightly more than 1 diameter from the anterior margin of the head. The scape is short and barely reaches the posterior lateral corner of the head. The pronotal shoulder is rounded, the promesonotal and metanotal sutures are marked on the dorsum of the mesosoma, but neither is greatly depressed when the mesosoma is viewed in profile. The propodeal spiracle is nearly circular. The petiole is narrow when viewed in profile and constricted toward the apex, forming a rounded surface. The subpetiolar process is broadly rounded and lobe-shaped.
Erect hairs are mostly short (about 0.1 mm in length, although hairs on the clypeus may be 0.25 mm in length) and are present on the mandibles, clypeus, dorsal and ventral surfaces of the head, antennal scapes, mesosoma, petiole, gaster and legs; appressed whitish pubescence is abundant on the head, mesosoma and gaster. The mandibles are smooth and moderately glossy with scattered punctures, the dorsum of the head is finely punctate and dull, the dorsum of the mesosoma has similar punctures, the sides are finely striate, most surfaces are at most weakly shining, the gaster is finely punctate and moderately shining.
Queen
From Mackay and Mackay (2010): The female is a small (total length 4 mm) reddish brown ant with lighter colored appendages. The mandible has six teeth with a small swelling near the basal border, which does not form a tooth. The anterior margin of the clypeus is convex and the clypeus has a transverse sharp ridge or carina, which is elevated over the anteclypeus. The head is narrowed anteriorly and posteriorly and the posterior border is slightly concave. The eye is large and separated from the anterior margin of the head by less than ½ of the greatest eye diameter (side view). The scape reaches or extends slightly past the posterior lateral corner of the head. The pronotal shoulder is without a carina; the propodeal spiracle is circular or elliptical. The propodeum is angulate between the dorsal face and the posterior face. The petiole is slender when viewed in profile and the subpetiolar process consists of a large broadly rounded lobe.
Erect hairs are present on the mandibles, dorsal and ventral surfaces of the head, the side of the head anterior to the eyes, the shaft of the scape, dorsum of the mesosoma, dorsum of the petiole, subpetiolar process and all surfaces of the gaster. The hairs on the legs are erect or suberect, with several on all surfaces of the tibiae. Most surfaces, except for the mandibles, are covered with an appressed pubescence, which is abundant to very abundant and obscures some of the surfaces, especially the dorsum of the head, dorsum of the mesosoma and dorsum of the gaster.
The mandibles are smooth and shining with scattered punctures, the remainder of the surfaces is dull and punctate; the gaster is weakly shining.
Male
From Mackay and Mackay (2010): The male (undescribed) is a small (total length 3.5 - 5.0 mm) medium brown to dark brown specimen with yellow to medium brown legs and antennae. The head length is 0.78 - 0.83 mm, the head width is 0.76 - 0.85 mm. The anterior margin of the clypeus is broadly concave; the eyes are large (maximum diameter 0.40 - 0.44 mm). The Mayrian furrows are present, but do not meet and the parapsidal sutures are well developed. The petiole is narrow when viewed in profile and the subpetiolar process consists of a broadly rounded lobe. Males apparently come in two forms: smaller medium brown specimen and larger dark brown specimen, but this conclusion is based on only a few specimens.
Erect hairs are sparse with a few on the dorsal surface of the head and clypeus, sides of the head anterior to the eyes, between the lateral ocelli, on the dorsum of the mesosoma, dorsum of the petiole, subpetiolar process and all surfaces of the gaster. Nearly all of the hairs on the legs are appressed. Appressed pubescence is sparse and mostly restricted to the head, dorsum of the mesosoma and all surfaces of the gaster. Most surfaces are punctate, but the mesosoma and gaster are weakly shining.
Karyotype
- See additional details at the Ant Chromosome Database.
 Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
- 2n = 12, karyotype = 10M+2A (Brazil) (Mariano et al., 2012) (as Pachycondyla gilberti).
- 2n = 14, karyotype = 12M+2AM (Brazil) (Mariano et al., 2007) (as Pachycondyla gilberti).
- n = 6, 2n = 12, karyotype = 10M+2SM (Brazil) (Correia et al., 2016).
- 2n = 12, karyotype = 10M+2SM (French Guiana) (Aguiar et al., 2020).
- n = 6, 2n = 12, karyotype = 12M (Brazil) (Mariano et al., 2011; Mariano et al., 2015).
Etymology
This species was named in honor of Father Columbano Gilbert, Professor of French and German of the Franciscan Seminary at Agudos, Brasil, 1914 – 2002, an “industrious discover of many myrmecological rarities” (Kempf, 1960a). (Mackay and Mackay 2010)
References
- Aguiar, H.J.A.C., Barros, L.A.C., Silveira, L.I., Petitclerc, F., Etienne, S., Orivel, J. 2020. Cytogenetic data for sixteen ant species from North-eastern Amazonia with phylogenetic insights into three subfamilies. Comparative Cytogenetics 14(1): 43–60 (doi:10.3897/CompCytogen.v14i1.46692).
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Brown, W. L., Jr. 1995a. [Untitled. Taxonomic changes in Pachycondyla attributed to Brown.] Pp. 302-311 in: Bolton, B. A new general catalogue of the ants of the world. Cambridge, Mass.: Harvard University Press, 504 pp. (page 305, Combination in Pachycondyla)
- Correia, J.P.S.O., Mariano, C S F., Delabie, J.H.C., Lacau, S., Costa, M.A. 2016. Cytogenetic analysis of Pseudoponera stigma and Pseudoponera gilberti (Hymenoptera: Formicidae: Ponerinae): a taxonomic approach. Florida Entomologist 99: 718-721.
- Esteves, F.A., Fisher, B.L. 2021. Corrieopone nouragues gen. nov., sp. nov., a new Ponerinae from French Guiana (Hymenoptera, Formicidae). ZooKeys 1074, 83–173 (doi:10.3897/zookeys.1074.75551).
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- Kempf, W. 1960a. Miscellaneous studies on Neotropical ants (Hymenoptera: Formicidae). Studia Entomologica 3:417-466.
- Lubertazzi, D. 2019. The ants of Hispaniola. Bulletin of the Museum of Comparative Zoology, 162(2), 59-210 (doi:10.3099/mcz-43.1).
- Mackay, W.P., Mackay, E.E. 2010. The systematics and biology of the New World ants of the genus Pachycondyla (Hymenoptera: Formicidae). Edwin Mellon Press, Lewiston.
- Mariano, C. d. S. F., Pompolo, S. d. G., Silva, J. G. & Delabie, J. H. C. 2011. Contribution of cytogenetics to the debate on the paraphyly of Pachycondyla spp. (Hymenoptera, Formicidae, Ponerinae). Psyche Volume 2012, Article ID 973897, 9 pp. (doi:10.1155/2012/973897).
- Mariano, C.S.F., Santos, I.S., Silva, J.G., Costa, M.A., Pompolo, S.G. 2015. Citogenética e evolução do cariótipo em formigas poneromorfas. In: Delabie, J.H.C., Feitosa, R.M., Serrao, J.E., Mariano, C.S.F., Majer, J.D. (eds) As formigas poneromorfas do Brasil, 1st edn. Ilhéus, Brasil, pp 102–125 (doi:10.7476/9788574554419.0010).
- Schmidt, C.A. & Shattuck, S.O. 2014. The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of ponerine ecology and behavior. Zootaxa 3817, 1–242 (doi:10.11646/zootaxa.3817.1.1).
- Wheeler, G. C.; Wheeler, J. 1971b. Ant larvae of the subfamily Ponerinae: second supplement. Ann. Entomol. Soc. Am. 6 64: 1197-1217 (page 1206, larva described)
References based on Global Ant Biodiversity Informatics
- Brandao, C.R.F. 1991. Adendos ao catalogo abreviado das formigas da regiao neotropical (Hymenoptera: Formicidae). Rev. Bras. Entomol. 35: 319-412.
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Franco W., N. Ladino, J. H. C. Delabie, A. Dejean, J. Orivel, M. Fichaux, S. Groc, M. Leponce, and R. M. Feitosa. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5): 509-543.
- Kempf W. W. 1960. Miscellaneous studies on Neotropical ants (Hymenoptera, Formicidae). Studia Entomologica (n.s.)3: 417-466.
- Kempf W. W. 1961. A survey of the ants of the soil fauna in Surinam (Hymenoptera: Formicidae). Studia Entomologica 4: 481-524.
- Kempf W. W. 1961. Nota preliminar sôbre a fauna das formigas de Agudos, S. P. (Hymenoptera: Formicidae). Revista Brasileira de Entomologia 10: 205-208.
- Kempf W. W. 1978. A preliminary zoogeographical analysis of a regional ant fauna in Latin America. 114. Studia Entomologica 20: 43-62.
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Mackay, W.P. and E.E. MacKay. 2010. The systematics and biology of the New World ants of the genus Pachycondyla (Hymenoptera: Formicidae). Edwin Mellen Press Lewiston, NY
- Marinho C. G. S., R. Zanetti, J. H. C. Delabie, M. N. Schlindwein, and L. de S. Ramos. 2002. Ant (Hymenoptera: Formicidae) Diversity in Eucalyptus (Myrtaceae) Plantations and Cerrado Litter in Minas Gerais, Brazil. Neotropical Entomology 31(2): 187-195.
- Mertl A. L., J. F. A. Traniello, K. Ryder Wilkie, and R. Constantino. 2012. Associations of two ecologically significant social insect taxa in the litter of an amazonian rainforest: is there a relationship between ant and termite species richness? Psyche doi:10.1155/2012/312054
- Pires de Prado L., R. M. Feitosa, S. Pinzon Triana, J. A. Munoz Gutierrez, G. X. Rousseau, R. Alves Silva, G. M. Siqueira, C. L. Caldas dos Santos, F. Veras Silva, T. Sanches Ranzani da Silva, A. Casadei-Ferreira, R. Rosa da Silva, and J. Andrade-Silva. 2019. An overview of the ant fauna (Hymenoptera: Formicidae) of the state of Maranhao, Brazil. Pap. Avulsos Zool. 59: e20195938.
- Ramos L. de S., C. G. S. Marinho, R. Zanetti, and J. H. C. Delabie. 2003. Impacto de iscas formicidas granuladas sobre a mirmecofauna não-alvo em eucaliptais segundo duas formas de aplicacação / Impact of formicid granulated baits on non-target ants in eucalyptus plantations according to two forms of application. Neotropical Entomology 32(2): 231-237.
- Ramos L. de S., R. Zanetti, C. G. S. Marinho, J. H. C. Delabie, M. N. Schlindwein, and R. P. Almado. 2004. Impact of mechanical and chemical weedings of Eucalyptus grandis undergrowth on an ant community (Hymenoptera: Formicidae). Rev. Árvore 28(1): 139-146.
- Scott-Santos, C.P., F.A. Esteves, C.R.F. Brandao. 2008. Catalogue of "Poneromorph" ant type specimens (Hymenoptera, Formicidae) deposited in the Museu de Zoologia da Universidade de Sao Paulo, Brazil. Papeis Avulsos de Zoologia 48(11):75-88.
- Siqueira de Castro F., A. B. Gontijo, P. de Tarso Amorim Castro, and S. Pontes Ribeiro. 2012. Annual and Seasonal Changes in the Structure of Litter-Dwelling Ant Assemblages (Hymenoptera: Formicidae) in Atlantic Semideciduous Forests. Psyche doi:10.1155/2012/959715
- Siqueira de Castro F., A. B. Gontijo, W. Duarte da Rocha, and S. Pontes Ribeiro. 2011. As comunidades de formigas de serapilheira nas florestas semidecíduas do Parque Estadual do Rio Doce, Minas Gerais. MG.BIOTA, Belo Horizonte 3(5): 5-24.

