Tetramorium lanuginosum
| Tetramorium lanuginosum | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Crematogastrini |
| Genus: | Tetramorium |
| Species group: | obesum |
| Species: | T. lanuginosum |
| Binomial name | |
| Tetramorium lanuginosum Mayr, 1870 | |
| Synonyms | |
| |
| Common Name | |
|---|---|
| Ikarige-shiwa-ari | |
| Language: | Japanese |
| Wooly ant | |
| Language: | English |
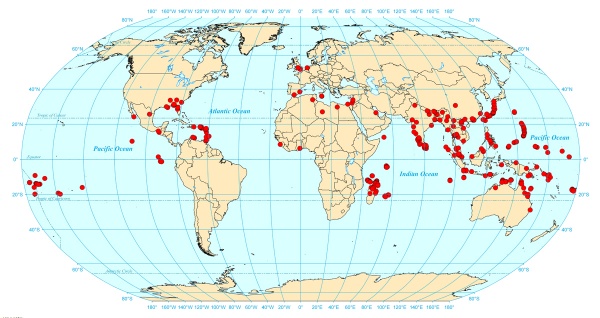
The wooly ant, Tetramorium lanuginosum, has long been recognized as a widespread tramp species dispersed through human commerce. Based on its distribution and those of its closest known relatives, T. lanuginosum appears to be native to tropical and subtropical East Asia and perhaps also northern Australia and western Oceania. Tetramorium lanuginosum appears to be particularly common on small islands, possibly due to reduced competition with dominant ants in these habitats. Recent first records of T. lanuginosum on many islands of Samoa, the Galapagos, Madagascar (and neighboring island groups), and the West Indies suggest that exotic populations of T. lanuginosum are expanding on numerous tropical islands. Nonetheless, it appears unlikely that T. lanuginosum will ever become a significant exotic pest species, except perhaps on small tropical islands (from the abstract of Wetterer 2010).
| At a Glance | • Highly invasive |
Identification
Monomorphic robust ants with a slow and steady gait. Workers possess a thick coat of soft erect hairs, some of which are branched into two or three tips.
Sharaf et al. (2017) - Worker. Head longer than broad with reticulate-rugose sculpture; anterior clypeal margin with small median impression; frontal carinae strongly developed; antennal scrobes well-developed with distinct margins; eyes of moderate size with 8–10 ommatidia in longest row; mesosoma convex in profile; metanotal groove absent; propodeal spines long and sharp; petiolar node rounded in profile; gaster smooth and shiny. Body pilosity profuse and relatively long, bifid and simple hairs. Body colour variable from pale brown to dark brown, gaster darker than body.
Keys including this Species
- Key to Afrotropical Tetramorium ericae osiris inezulae gabonensis species groups
- Key to Arabian Tetramorium
- Key to Australian Tetramorium Species
- Key to Micronesian Ants
- Key to Tetramorium of Hispaniola
- Key to Tetramorium of India
- Key to US Tetramorium species
- Key to workers of the Socotra Archipelago, Yemen
Distribution
Modified from Wetterer (2010): Tetramorium lanuginosum is widespread in tropical and subtropical parts of Asia, Australia, and Oceania. Outside of this region most records are concentrated in three areas: Madagascar and neighboring islands, the Galapagos, and the Eastern Caribbean. Records for these island groups are largely records from the past ten years (c. 2000-2010). Other more scattered reports including records from tropical Africa, the Mediterranean, Mexico, and the southeastern US, Northern Europe (three sites - Kew Gardens, the Birmingham Botanical Garden, and the Dudley Zoo) and one site in the Netherlands (an indoor record in Amsterdam; DE JONGE 1985).
It is believed Tetramorium lanuginosum is native to tropical Asia. The species occurs over a seemingly continuous range from India, through tropical and subtropical East Asia, to northern Australia, and thus is probably native to much of this region, and perhaps even to parts of western Oceania, e.g., the Solomon Islands, Palau, and the Mariana Islands. (Wetterer 2010)
Latitudinal Distribution Pattern
Latitudinal Range: 32.812778° to -28.398°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Afrotropical Region: Comoros, Saudi Arabia, Socotra Archipelago, Yemen.
Australasian Region: Australia (type locality).
Indo-Australian Region: American Samoa, Borneo, Fiji, Guam, Indonesia (type locality), Kiribati, Krakatau Islands, Malaysia, Marshall Islands, Micronesia (Federated States of), New Guinea, Philippines, Samoa, Singapore, Solomon Islands, Tokelau, Tonga, Wallis and Futuna Islands.
Malagasy Region: Madagascar, Mauritius, Mayotte, Réunion, Seychelles.
Nearctic Region: United States.
Neotropical Region: Aruba, Barbados, Dominican Republic, Ecuador, Galapagos Islands, Greater Antilles, Mexico, Netherlands Antilles, Puerto Rico.
Oriental Region: Bangladesh, Cambodia, India, Laos, Myanmar, Nepal, Nicobar Island, Sri Lanka, Thailand, Vietnam.
Palaearctic Region: China, Cyprus, Iberian Peninsula, Israel, Japan, Malta, Oman, Spain, Türkiye.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Distribution records of Tetramorium lanuginosum as provided by James Wetterer, 2010.
Biology
Regional Notes
Malagasy region
Hita Garcia and Fisher (2011) - In the Malagasy region T. lanuginosum is relatively widespread. The species can be found in many locations of Northern Madagascar and on most of its surrounding island systems, e.g. the Comoros, Mayotte, Mauritius, Seychelles, and Reunion. Wetterer (2010) points out that T. lanuginosum might have reached the Malagasy region recently because almost all records of the species are dated after 2001. We only tentatively agree with this point of view since T. mauricei, now a synonym of T. lanuginosum, was collected in 1942 from Mauritius and later described by Donisthorpe (1946). Another reason to be cautious is that most collecting in the Malagasy region has been performed in the last two decades; thus, large numbers of fresher specimens does not necessarily prove that the species was not abundant previously. Nevertheless, T. lanuginosum was not mentioned from Mauritius by Ward (1990) so it is possible tat the species was already present in the area but spread over the last one or two decades.
Oman
Sharaf et al. (2018) - This species was found nesting under a stone next to a date palm tree where soil was moist. It was also found in leaf litter under a mango tree.
Yemen
Sharaf et al. (2017) - A nest was found under the trunk of a fallen, dead palm tree where the soil was moist and rich in decaying organic material. Individuals were collected from dry soil next to a date palm tree. Several specimens were observed foraging on the ground where the soil was moist. Other workers were found foraging on the ground near a small flowing stream where the soil was sandy and moist. This species seems likely to have been introduced to Socotra through commerce.
Castes
Additional images can be found on the lanuginosum category page.
Worker
   
| |
| . | |
Images from AntWeb
   
| |
| Syntype of Triglyphothrix tricolor. Worker. Specimen code castype06959-01. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Worker. Specimen code casent0060515. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Worker. Specimen code casent0101229. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by MHNG, Geneva, Switzerland. |
   
| |
| Holotype of Tetramorium lanuginosum. Worker. Specimen code casent0102356. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by NHMUK, London, UK. |
Queen
Images from AntWeb
   
| |
| Syntype of Triglyphothrix tricolor. Queen (alate/dealate). Specimen code castype06959-02. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Queen (alate/dealate). Specimen code casent0060445. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Queen (alate/dealate). Specimen code casent0103336. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- lanuginosum. Tetramorium lanuginosum Mayr, 1870b: 976 (w.) INDONESIA (Java). Viehmeyer, 1916a: 140 (q.); Imai, Baroni Urbani, et al. 1984: 8 (k.). Combination in Triglyphothrix: Emery, 1891b: 4 (footnote); in Tetramorium: Bolton, 1985: 247. Senior synonym of australis, ceramensis, felix, flavescens, laevidens, mauricei, orissana, striatidens, tricolor: Bolton, 1976: 350. See also: Hita Garcia & Fisher, 2011: 27.
- striatidens. Tetramorium obesum r. striatidens Emery, 1889b: 501 (w.) MYANMAR. Wheeler, G.C. & Wheeler, J. 1973b: 78 (l.). Combination in Triglyphothrix: Emery, 1891b: 4. Raised to species: Forel, 1903a: 704. Junior synonym of lanuginosum: Bolton, 1976: 350.
- laevidens. Triglyphothrix striatidens var. laevidens Forel, 1900e: 284 (w.) MEXICO. Junior synonym of lanuginosum: Bolton, 1976: 350.
- australis. Triglyphothrix striatidens var. australis Forel, 1902h: 449 (w.q.) AUSTRALIA. Junior synonym of lanuginosum: Bolton, 1976: 350.
- orissana. Triglyphothrix striatidens r. orissana Forel, 1902c: 239 (w.) INDIA. Raised to species: Bingham, 1903: 174. Subspecies of striatidens: Emery, 1924d: 274. Junior synonym of lanuginosum: Bolton, 1976: 350.
- ceramensis. Triglyphothrix ceramensis Stitz, 1912: 506 (w.) INDONESIA (Seram I.). Junior synonym of lanuginosum: Bolton, 1976: 350.
- felix. Triglyphothrix striatidens var. felix Forel, 1912k: 160 (w.) SEYCHELLES IS. Junior synonym of lanuginosum: Bolton, 1976: 350.
- flavescens. Triglyphothrix striatidens var. flavescens Wheeler, W.M. 1929g: 55 (w.) SINGAPORE. [Unresolved junior secondary homonym of flavescens Emery, above.] Junior synonym of lanuginosum: Bolton, 1976: 350.
- mauricei. Triglyphothrix mauricei Donisthorpe, 1946c: 778 (w.) MAURITIUS. Junior synonym of lanuginosum: Bolton, 1976: 350.
- tricolor. Triglyphothrix tricolor Donisthorpe, 1948g: 136 (w.q.) NEW GUINEA. Junior synonym of lanuginosum: Bolton, 1976: 350.
Type Material
Hita Garcia and Fisher (2011 ):
- Tetramorium lanuginosum. Holotype worker, INDONESIA, Java, Batavia (Naturhistorisches Museum Wien, Vienna) [examined].
- Tetramorium obesum r. striatidens Syntype workers, BURMA, Bhamo, VII.1886 (L. Fea) (Musee d'Histoire Naturelle Genève) [examined].
- Triglyphothrix striatidens var. laevidens Syntype workers, MEXICO (Musee d'Histoire Naturelle Genève) [examined].
- Triglyphothrix striatidens r. australis Syntype workers, queens, AUSTRALIA, Queensland, Mackay (Turner) (Musee d'Histoire Naturelle Genève; Museum of Comparative Zoology) [partly examined].
- Triglyphothrix striatidens r. orissana Syntype workers, INDIA, Orissa (Taylor) (Musee d'Histoire Naturelle Genève) [examined].
- Triglyphothrix striatidens var. felix Syntype workers, SEYCHELLES, Felicite, Silhoutte, Mare aux Cochons, 1908 (H. Scott) (Musee d'Histoire Naturelle Genève; The Natural History Museum) [examined].
- Triglyphothrix ceramensis Holotype worker, INDONESIA, Seram Island (holotype location unknown).
- Triglyphothrix striatidens var. flavescens Syntype workers, SINGAPORE, Johore, 2.II.1925 (F. Silvestri) (Museum of Comparative Zoology) [not examined].
- Triglyphothrix mauricei Holotype worker, MAURITIUS, Rose Hill, 1942 (R. Mamet) (The Natural History Museum) [examined].
- Triglyphothrix tricolor Paratype workers, queens, NEW GUINEA, Maffin Bay, 17. & 20.VI.1944 (E.S. Ross) (The Natural History Museum, California Academy of Sciences) [examined].
- Tetramorium lanuginosum: Holotype, worker, Jakarta (as Batavia), Indonesia, Naturhistorisches Museum Wien, Vienna.
- Triglyphothrix (Xiphomyrmex) striatidens australis: Syntype, worker(s), Mackay, Queensland, Australia, Australian National Insect Collection.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Hita Garcia and Fisher (2011 ) - HL 0.595–0.655 (0.626); HW 0.560–0.630 (0.590); SL 0.400–0.475 (0.438); EL 0.120–0.150 (0.137); PW 0.380–0.440 (0.418); WL 0.630–0.720 (0.683); PSL 0.155–0.205 (0.172); PTL 0.185–0.220 (0.200); PTH 0.190–0.215 (0.203); PTW 0.180–0.230 (0.210); PPL 0.170–0.215 (0.194); PPH 0.160–0.195 (0.179); PPW 0.190–0.230 (0.216); CI 92–98 (94); SI 71–76 (74); OI 21–25 (23); PSLI 25–31 (28); PeNI 47–54 (50); LPeI 93–105 (99); DpeI 97–110 (104); PpNI 48–55 (52); LPpI 100–116 (109); DPpI 105–121 (111); PPI 98–110 (103) (20 measured).
Head longer than wide (CI 92–98). Anterior clypeal margin with small but distinct median impression. Frontal carinae strongly developed, curving down ventrally between posterior eye level and posterior margin of head to form posterior and ventral margins of antennal scrobe. Antennal scrobes well-developed and broad, usually with distinct margin all around. Antennal scapes relatively short, fitting well within antennal scrobe (SI 71–76). Eyes of moderate size (OI 21–25), with 8 to 10 ommatidia in longest row. Mesosoma convex in profile, sides rounding smoothly onto dorsum. Metanotal groove absent. Propodeal spines medium-sized to long and spinose (PSLI 25-31). Propodeal lobes small, triangular, and acute. Node of petiole rounded nodiform in profile, anterodorsal angle well-defined but not sharply angulate, situated much higher than rounded posterodorsal angle, causing dorsum to taper backward to posterior face; in dorsal view roughly as long as wide to weakly wider than long (DPeI 97–110), in lateral view roughly as long as high (LPeI 93–105). Postpetiole in lateral view rounded and much less voluminous than petiolar node, usually weakly longer than high (LPpI 100–116); in dorsal view postpetiole roughly as wide to weakly wider than petiole (PPI 98–110), and weakly wider than long (DPpI 105–121). Mandibles generally longitudinally striate, sometimes weakly developed. Clypeus usually longitudinally rugose, median ruga always developed with 1 to 2 weaker rugae at each side. Head reticulate-rugose, cephalic dorsum between frontal carinae dorsally more reticulate, close to the posterior clypeal margin often more longitudinally rugose. Ground sculpturation generally very weak to absent. Mesosoma and waist segments also reticulate-rugose, without any conspicuous ground sculpture. Gaster completely unsculptured, smooth, and shiny. Body pilosity usually very dense and relatively long but variable, generally all dorsal surfaces of head, mesosoma, waist segments, and gaster with numerous long bifid and simple hairs, usually a mixture of both present with either bifid or simple pilosity predominant, but both types always present; rarely some trifid hairs also present, though almost never on first gastral tergite. Variation in pilosity often observable within same series from same collection event. Frontal carinae and leading edges of antennal scapes with elongate, simple, standing hairs. Tibiae with relatively long suberect to erect standing hairs. Colouration variable, light brown to dark brown, gaster often darker than remaining body.
Karyotype
- 2n = 14, karyotype = 12M+2A (India) (Imai et al., 1984) (as Triglyphothrix lanuginosa).
References
- Agavekar, G., Hita Garcia, F., Economo, E.P. 2017. Taxonomic overview of the hyperdiverse ant genus Tetramorium Mayr (Hymenoptera, Formicidae) in India with descriptions and X-ray microtomography of two new species from the Andaman Islands. PeerJ 5:e3800 (DOI 10.7717/peerj.3800).
- Bharti, H. & Kumar, R. 2012. Taxonomic studies on genus Tetramorium Mayr (Hymenoptera, Formicidae) with report of two new species and three new records including a tramp species from India with a revised key. ZooKeys. 207:11-35. doi:10.3897/zookeys.207.3040
- Blard, F., Dorow, W.-H.-O., Delabie, J. H. C. 2003. Les Fourmis de l’île de la Réunion (Hymenoptera, Formicidae). Bulletin de La Société Entomologique de France, 108(2), 127–137 (doi:10.3406/bsef.2003.16939).
- Bolton, B. 1976. The ant tribe Tetramoriini (Hymenoptera: Formicidae). Constituent genera, review of smaller genera and revision of Triglyphothrix Forel. Bull. Br. Mus. (Nat. Hist.) Entomol. 34: 281-379 (page 350, Senior synonym of australis, ceramensis, felix, flavescens, laevidens, mauricei, orissana, striatidens and tricolor.)
- Bolton, B. 1985. The ant genus Triglyphothrix Forel a synonym of Tetramorium Mayr. (Hymenoptera: Formicidae). J. Nat. Hist. 19: 243-248 (page 247, Combination in Tetramorium)
- Brassard, F., Leong, C.-M., Chan, H.-H., Guénard, B. 2021. High diversity in urban areas: How comprehensive sampling reveals high ant species richness within one of the most urbanized regions of the world. Diversity 13, 358 (doi:10.3390/d13080358).
- Casiraghi, A., Espadaler, X., Pérez Hidalgo, N., Gómez, K. 2020. Two additions to the Iberian myrmecofauna: Crematogaster inermis Mayr, 1862, a newly established, tree-nesting species, and Trichomyrmex mayri (Forel, 1902), an emerging exotic species temporarily nesting in Spain (Hymenoptera, Formicidae). Journal of Hymenoptera Research 78, 57–68 (doi:10.3897/jhr.78.51858).
- Collingwood, C. A., Pohl, H., Guesten, R., Wranik, W. and van Harten, A. 2004. The ants (Insecta: Hymenoptera: Formicidae) of the Socotra Archipelago. Fauna of Arabia. 20:473-495.
- Dekoninck, W., Wauters, N., Delsinne, T. 2019. Capitulo 35. Hormigas invasoras en Colombia. Hormigas de Colombia.
- Dendup, K.C., Dorji, C., Dhadwal, T., Bharti, H., Pfeiffer, M. 2021. A preliminary checklist of ants from Bhutan. Asian Myrmecology 14, e014005 (doi:10.20362/am.014005).
- Emery, C. 1891c. Exploration scientifique de la Tunisie. Zoologie. - Hyménoptères. Révision critique des fourmis de la Tunisie. Paris: Imprimerie Nationale, iii + 21 pp. (page 4, Combination in Triglyphothrix)
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- Hasin, S., Tasen, W. 2020. Ant community composition in urban areas of Bangkok, Thailand. Agriculture and Natural Resources 54: 507-514 (doi:10.34044/j.anres.2020.54.5.07).
- Hasin, S., Tasen, W., Ohashi, M., Boonriam, W., Yamada, A. 2021. Yellow crazy ants (Anoplolepis gracilipes [Smith, F., 1857]: Hymenoptera: Formicidae) threaten community of ground-dwelling arthropods in dry evergreen forests of Thailand. Agriculture and Natural Resources 55: 634-643 (doi:10.34044/j.anres.2021.55.4.14).
- Herrera, H.W., Baert, L., Dekoninck, W., Causton, C.E., Sevilla, C.R., Pozo, P., Hendrickx, F. 2020. Distribution and habitat preferences of Galápagos ants (Hymenoptera: Formicidae). Belgian Journal of Entomology, 93: 1–60.
- Heterick, B.E. 2021. A guide to the ants of Western Australia. Part I: Systematics. Records of the Western Australian Museum, Supplement 86, 1-245 (doi:10.18195/issn.0313-122x.86.2021.001-245).
- Heterick, B.E. 2022. A guide to the ants of Western Australia. Part II: Distribution and biology. Records of the Western Australian Museum, supplement 86: 247-510 (doi:10.18195/issn.0313-122x.86.2022.247-510).
- Hita Garcia and Fisher. 2011. The ant genus Tetramorium Mayr (Hymenoptera: Formicidae) in the Malagasy region – introduction, definition of species groups, and revision of the T. bicarinatum, T. obesum, T. sericeiventre and T. tosii species groups. Zootaxa. 3039: 1-72.
- Hoffmann, B., Eldridge, J., Marston, C. 2023. The first eradication of an exotic ant species from the entirety of Australia: Pheidole fervens. Management of Biological Invasions, 14(4), 619–624 (doi:10.3391/mbi.2023.14.4.03).
- Hosoishi, S., Rahman, M. M., Heng, S. 2022. Exotic ants (Hymenoptera: Formicidae) of Cambodia. Far Eastern Entomologist 460, 15–24 (doi:10.25221/fee.460.3).
- Imai, H. T.; Baroni Urbani, C.; Kubota, M.; Sharma, G. P.; Narasimhanna, M. H.; Das, B. C.; 1984. Karyological survey of Indian ants. Jpn. J. Genet. 59: 1-32 (page 8, karyotype described)
- Imai, H.T., Kihara, A., Kondoh, M., Kubota, M., Kuribayashi, S., Ogata, K., Onoyama, K., Taylor, R.W., Terayama, M., Yoshimura, M., Ugawa, Y. 2003. Ants of Japan. 224 pp, Gakken, Japan.
- Karaman, C., Kıran, K. 2018. New tramp ant species for Turkey: Tetramorium lanuginosum Mayr (Hymenoptera: Formicidae). Trakya University Journal of Natural Sciences 19(1), e1-e4 (doi:10.23902/trkjnat.340008).
- Katayama, M., Tsuji, K. 2010. Habitat differences and occurrence of native and exotic ants on Okinawa Island. Entomological Science 13, 425–429 (doi:10.1111/j.1479-8298.2010.00400.x).
- Khachonpisitsak, S., Yamane, S., Sriwichai, P., Jaitrong, W. 2020. An updated checklist of the ants of Thailand (Hymenoptera, Formicidae). ZooKeys 998, 1–182 (doi:10.3897/zookeys.998.54902).
- Kiran, K., Karaman, C. 2020. Additions to the ant fauna of Turkey (Hymenoptera, Formicidae). Zoosystema 42(18), 285-329 (doi:10.5252/zoosystema2020v42a18).
- Lee, C.-C., Weng, Y.-M., Lai, L.-C., Suarez, A.V., Wu, W.-J., Lin, C.-C., Yang, C.-C.S. 2020. Analysis of recent interception records reveals frequent transport of arboreal ants and potential predictors for ant invasion in Taiwan. Insects 11, 356 (doi:10.3390/INSECTS11060356).
- Lubertazzi, D. 2019. The ants of Hispaniola. Bulletin of the Museum of Comparative Zoology, 162(2), 59-210 (doi:10.3099/mcz-43.1).
- Mayr, G. 1870b. Neue Formiciden. Verh. K-K. Zool.-Bot. Ges. Wien 20: 939-996 (page 972,976, worker described)
- Nooten, S.S., Lee, R.H., Guénard, B. 2021. Evaluating the conservation value of sacred forests for ant taxonomic, functional and phylogenetic diversity in highly degraded landscapes. Biological Conservation 261, 109286 (doi:10.1016/j.biocon.2021.109286).
- Oussalah, N., Marniche, F., Espadaler, X., Biche, M. 2019. Exotic ants from the Maghreb (Hymenoptera, Formicidae) with first report of the hairy alien ant Nylanderia jaegerskioeldi (Mayr) in Algeria. Arxius de Miscel·lània Zoològica, 45–58 (doi:10.32800/amz.2019.17.0045).
- Rosas-Mejía, M., Guénard, B., Aguilar-Méndez, M. J., Ghilardi, A., Vásquez-Bolaños, M., Economo, E. P., Janda, M. 2021. Alien ants (Hymenoptera: Formicidae) in Mexico: the first database of records. Biological Invasions 23(6), 1669–1680 (doi:10.1007/s10530-020-02423-1).
- Sanetra, M., Buschinger, A. 2000. Phylogenetic relationships among social parasites and their hosts in the ant tribe Tetramoriini (Hymenoptera: Formicidae). European Journal of Entomology 97: 95-117.
- Schifani, E., Csősz, S., Viviano, R., Alicata, A. 2021. Ant diversity on the largest Mediterranean islands: on the presence or absence of 28 species in Sicily (Hymenoptera, Formicidae). Natural History Sciences 8, 55–70 (doi:10.4081/nhs.2021.532).
- Sharaf, M. R. , B. L. Fisher, H. M. Al Dhafer, A. Polaszek and A. S. Aldawood. 2018. Additions to the ant fauna (Hymenoptera: Formicidae) of Oman: an updated list, new records and a description of two new species. Asian Myrmecology. 9:e010004; 1-38. doi:10.20362/am.010004
- Sharaf, M. R., Wetterer, J. K., Mohamed, A. A., Aldawood, A. S. 2022. Faunal composition, diversity, and distribution of ants (Hymenoptera: Formicidae) of Dhofar Governorate, Oman, with updated list of the Omani species and remarks on zoogeography. European Journal of Taxonomy 838: 1-106 (doi:10.5852/ejt.2022.838.1925).
- Sharaf, M.R., Abdel-Dayem, M.S., Mohamed, A.A., Fisher, B.L., Aldawood, A.S. 2020. A preliminary synopsis of the ant fauna (Hymenoptera: Formicidae) of Qatar with remarks on the zoogeography. Annales Zoologici 70: 533-560 (doi:10.3161/00034541anz2020.70.4.005).
- Sharaf, M.R., Fisher, B.L., Collingwood, C.A., Aldawood, A.S. 2017. Ant fauna (Hymenoptera: Formicidae) of the Socotra Archipelago (Yemen): zoogeography, distribution and description of a new species. Journal of Natural History 51, 317–378 (DOI 10.1080/00222933.2016.1271157).
- Subedi, I.P., Budha, P.B., Bharti, H., Alonso, L. 2020. An updated checklist of Nepalese ants (Hymenoptera, Formicidae). ZooKeys 1006, 99–136 (doi:10.3897/zookeys.1006.58808).
- Tseng, S.-P. 2020. Evolutionary history of a global invasive ant, Paratrechina longicornis (Dissertation_全文 ). Ph.D. thesis, Kyoto University.
- Tseng, S.-P., Hsu, P.-W., Lee, C.-C., Wetterer, J.K., Hugel, S., Wu, L.-H., Lee, C.-Y., Yoshimura, T., Yang, C.-C.S. 2020. Evidence for common horizontal transmission of Wolbachia among ants and ant crickets: Kleptoparasitism added to the list. Microorganisms 8, 805. (doi:10.3390/MICROORGANISMS8060805).
- Viehmeyer, H. 1916a [1915]. Ameisen von Singapore. Beobachtet und gesammelt von H. Overbeck. Arch. Naturgesch. (A) 81(8): 108-168 (page 140, queen described)
- Wang, W.Y., Soh, E.J.Y., Yong, G.W.J., Wong, M.K.L., Benoit Guénard, Economo, E.P., Yamane, S. 2022. Remarkable diversity in a little red dot: a comprehensive checklist of known ant species in Singapore (Hymenoptera: Formicidae) with notes on ecology and taxonomy. Asian Myrmecology 15: e015006 (doi:10.20362/am.015006).
- Wetterer, J.K. 2010. Worldwide spread of the wooly ant, Tetramorium lanuginosum (Hymenoptera: Formicidae). Myrmecological News 13: 81-88.
- Wetterer, J.K. 2021. Ants (Hymenoptera, Formicidae) of St. Vincent, West Indies. Sociobiology 68, e6725 (doi:10.13102/sociobiology.v68i2.6725).
- Yamane, S., Hosoishi, S., Ito, F. 2022. Japanese Tetramorium queens: identification key and species diagnoses (Hymenoptera, Formicidae, Myrmicinae). ZooKeys 1084: 43–64 (doi:10.3897/zookeys.1084.69767).
References based on Global Ant Biodiversity Informatics
- Abe T., S. Yamane, and K. Onoyama. Ants collected on the Krakatau Islands 100 years after the great eruptions. Biogeography 14: 65-75.
- Andersen A. N., J. C. Z. Woinarski, and B. Hoffman. 2004. Biogeography of the ant fauna of the Tiwi Islands, in northern Australia's moonsoonal tropics. Australian Journal of Zoology 52: 97-110.
- Andersen A. N., R. R. Ribbons, M. Pettit, and C. L. Parr. 2014. Burning for biodiversity: highly resilient ant communities respond only to strongly contrasting fire regimes in Australias seasonal tropics. Journal of Applied Ecology 51: 14061413.
- Andersen A. N., and S. C. Morrison. 1998. Myrmecochory in Australia's seasonal tropics: effects of disturbance on distnce dispersal. Australian Journal of Ecology 23: 483-491.
- Andersen, Alan N., John C.Z. Woinarski and Ben D. Hoffman. 2004. Biogeography of the ant fauna of the Tiwi Islands, in northern Australia's monsoonal tropics. Australian Journal of Zoology 52: 97-110.
- Asfiya W., R. Ubaidillah, and Sk. Yamane. 2008. Ants (Hymenoptera: Formicidae) of the Krakataus, and Sebesi and Sebuku islands. Treubia 36: 1-9.
- Basu P., N. Tak, and A. K. Sanyal. 2013. Ants (insecta: Hymenoptera: Formicidae) of Bethuadahari wildlife sanctuary, Nadia, West Bengal, India. Rec. zool, Surv. India: 113(4): 17-22.
- Bharti H., Y. P. Sharma, M. Bharti, and M. Pfeiffer. 2013. Ant species richness, endemicity and functional groups, along an elevational gradient in the Himalayas. Asian Myrmecology 5: 79-101.
- Bolton B. 1976. The ant tribe Tetramoriini (Hymenoptera: Formicidae). Constituent genera, review of smaller genera and revision of Triglyphothrix Forel. Bulletin of the British Museum (Natural History). Entomology 34:281-379.
- Borowiec L., and S. Salata. 2019. Next step in the invasion: Trichomyrmex mayri (Forel, 1902) new to the Philippines (Hymenoptera: Formicidae). Annals of the Upper Silesian Museum in Bytom Entomology 28(3): 1-3.
- Chapman, J. W., and Capco, S. R. 1951. Check list of the ants (Hymenoptera: Formicidae) of Asia. Monogr. Inst. Sci. Technol. Manila 1: 1-327
- Clouse R. M. 2007. The ants of Micronesia (Hymenoptera: Formicidae). Micronesica. 39: 171-295.
- Clouse, R.M. 2007. The ants of Micronesia (Hymenoptera: Formicidae), Micronesica 39(2): 171-295.
- Crawley W. C. 1915. Ants from north and south-west Australia (G. F. Hill, Rowland Turner) and Christmas Island, Straits Settlements. - Part II. Annals and Magazine of Natural History (8)15: 232-239.
- Dad J. M., S. A. Akbar, H. Bharti, and A. A. Wachkoo. 2019. Community structure and ant species diversity across select sites ofWestern Ghats, India. Acta Ecologica Sinica 39: 219–228.
- Dahl F. 1901. Das Leben der Ameisen im Bismarck-Archipel, nach eigenen Beobachtungen vergleichend dargestellt. Mitt. Zool. Mus. Berl. 2: 1-70.
- Donisthorpe H. 1945. A new species of Triglyphothrix Forel (Hym., Formicidae) from Uganda with some notes on the genus. Entomologist's Monthly Magazine 81: 76-77.
- Donisthorpe H. 1948. A fourth instalment of the Ross Collection of ants from New Guinea. Annals and Magazine of Natural History (12)1: 131-143.
- Donisthorpe H. 1949. A sixth instalment of the Ross Collection of ants from New Guinea. Annals and Magazine of Natural History (12)1: 744-759.
- Donisthorpe, Horace. 1935. The Ants of Christmas Island. Annals and Magazine of Natural History. 25:629-635.
- Donisthorpe, Horace. 1935. The Ants of Christmas Island. Annals and Magazine of Natural History. Ser. 10: xv. 629-635.
- Eguchi K.; Bui T. V.; Yamane S. 2011. Generic synopsis of the Formicidae of Vietnam (Insecta: Hymenoptera), part I Myrmicinae and Pseudomyrmecinae. Zootaxa 2878: 1-61.
- Emery C. 1893. Formicides de l'Archipel Malais. Revue Suisse de Zoologie 1: 187-229.
- Emery C. 1893. Voyage de M. E. Simon à l'île de Ceylan (janvier-février 1892). Formicides. Annales de la Société Entomologique de France 62: 239-258.
- Emery C. Formiche raccolte da Elio Modigliani in Sumatra, Engano e Mentawei. Annali del Museo Civico di Storia Naturale 40: 661-722.
- Emery, C. "Formiche raccolte da Elio Modigliani in Sumatra, Engano e Mentawei." Annali del Museo Civico di Storia Naturale Giacomo Doria (Genova) (2) 20, no. 40 (1900): 661-722.
- Field Museum Collection, Chicago, Illinois (C. Moreau)
- Fisher J., L. Beames, B. J. Rangers, N. N. Rangers, J. Majer, and B. Heterick. 2014. Using ants to monitor changes within and surrounding the endangered Monsoon Vine Thickets of the tropical Dampier Peninsula, north Western Australia. Forest Ecology and Management 318: 7890.
- Floren A., W. Wetzel, and M. Staab. 2013. The contribution of canopy species to overall ant diversity (Hymenoptera: Formicidae) in temperate and tropical ecosystems. Myrmecological News 19: 65-74.
- Fontanilla A. M., A. Nakamura, Z. Xu, M. Cao, R. L. Kitching, Y. Tang, and C. J. Burwell. 2019. Taxonomic and functional ant diversity along tropical, subtropical, and subalpine elevational transects in southwest China. Insects 10, 128; doi:10.3390/insects10050128
- Forel A. 1901. Formiciden aus dem Bismarck-Archipel, auf Grundlage des von Prof. Dr. F. Dahl gesammelten Materials. Mitt. Zool. Mus. Berl. 2: 4-37.
- Forel A. 1903. Les Formicides de l'Empire des Indes et de Ceylan. Part X. J. Bombay Nat. Hist. Soc. 14: 679-715.
- Forel A. 1913k. Wissenschaftliche Ergebnisse einer Forschungsreise nach Ostindien ausgeführt im Auftrage der Kgl. Preuss. Akademie der Wissenschaften zu Berlin von H. v. Buttel-Reepen. II. Ameisen aus Sumatra, Java, Malacca und Ceylon. Gesammelt von Herrn Prof. Dr. v. Buttel-Reepen in den Jahren 1911-1912. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 36:1-148.
- Forel, A. 1908. Fourmis de Ceylan et d'Égypte récoltées par le Prof. E. Bugnion. Lasius carniolicus. Fourmis de Kerguelen. Pseudandrie? Strongylognathus testaceus. Bull. Soc. Vaudoise Sci. Nat. 44: 1-22
- Framenau V.W., and M.L. Thomas. 2008. Ants of Christmas Island (Indian Ocean); identification and distribution. Records of the Western Australian Museum 25: 45-85.
- Ghosh S. N., S. Sheela, B. G. Kundu, S. Roychowdhury, and R. N. Tiwari. 2006. Insecta: Hymenoptera: Formicidae. Pp. 369-398 in: Alfred, J. R. B. (ed.) 2006. Fauna of Arunachal Pradesh. (Part -2). [State Fauna Series 13.]. New Delhi: Zoological Survey of India, iv + 518 pp.
- Guénard B., and R. R. Dunn. 2012. A checklist of the ants of China. Zootaxa 3558: 1-77.
- Hita Garcia F., and B. L. Fisher. 2011. The ant genus Tetramorium Mayr (Hymenoptera: Formicidae) in the Malagasy regionintroduction, definition of species groups, and revision of the T. bicarinatum, T. obesum, T. sericeiventre and T. tosii species groups. Zootaxa 3039: 1-72.
- Hita García, F., and B. L. Fisher. "The ant genus Tetramorium Mayr (Hymenoptera: Formicidae) in the Malagasy regiontaxonomy of the T. bessonii, T. bonibony, T. dysalum, T. marginatum, T. tsingy, and T. weitzeckeri species groups." Zootaxa 3365 (2012): 1-123.
- Hu C.-H. 2006. Indigenized conservation and biodiversity maintenance on Orchid Island. PhD Thesis, graduate school of the University of Minnesota. 150 pages.
- Huong N. T. T., P. V. Sang, and B. T. Viet. 2015. A preliminary study on diversity of ants (Hymenoptera: Formicidae) at Hon Ba Nature Reserve. Environmental Scientific Conference 7: 614-620.
- Imai H. T., C. Baroni Urbani, M. Kubota, G. P. Sharma, M. H. Narasimhanna, B. C. Das, A. K. Sharma, A. Sharma, G. B. Deodikar, V. G. Vaidya, and M. R. Rajasekarasetty. 1984. Karyological survey of Indian ants. Japanese Journal of Genetics 59: 1-32.
- Ito, F.; Yamane, S.; Eguchi, K.; Noerdjito, W. A.; Kahono, S.; Tsuji, K.; Ohkawara, K.; Yamauchi, K.; Nishida, T.; Nakamura, K. 2001. Ant species diversity in the Bogor Botanic Garden, West Java, Indonesia, with descriptions of two new species of the genus Leptanilla (Hymenoptera, Formicidae). Tropics 10:379-404.
- Jaitrong W., B. Guenard, E. P. Economo, N. Buddhakala, and S. Yamane. 2016. A checklist of known ant species of Laos (Hymenoptera: Formicidae). Asian Myrmecology 8: 1-32. DOI: 10.20362/am.008019
- Jaitrong W., and T. Ting-Nga. 2005. Ant fauna of Peninsular Botanical Garden (Khao Chong), Trang Province, Southern Thailand (Hymenoptera: Formicidae). The Thailand Natural History Museum Journal 1(2): 137-147.
- Jaitrong W.; Nabhitabhata, J. 2005. A list of known ant species of Thailand. The Thailand Natural History Museum Journal 1(1): 9-54.
- Janda M., G. D. Alpert, M. L. Borowiec, E. P. Economo, P. Klimes, E. Sarnat, and S. O. Shattuck. 2011. Cheklist of ants described and recorded from New Guinea and associated islands. Available on http://www.newguineants.org/. Accessed on 24th Feb. 2011.
- Karavaiev V. 1935. Neue Ameisen aus dem Indo-Australischen Gebiet, nebst Revision einiger Formen. Treubia 15: 57-118.
- Kishimoto-Yamata K., F. Hyodo, M. Matsuoka, Y. Hashimoto, M. Kon, T. Ochi, S. Yamane, R. Ishii, and T. Itioka. 2012. Effects of remnant primary forests on ant and dung beetle species diversity in a secondary forest in Sarawak, Malaysia. Journal of Insect Conservation DOI 10.1007/s10841-012-9544-6
- Leong C. M., S. F. Shiao, and B. Guenard. 2017. Ants in the city, a preliminary checklist of Formicidae (Hymenoptera) in Macau, one of the most heavily urbanized regions of the world. Asian Myrmecology 9: e009014.
- Li Qiao, Chen You-qing, Guo Xiao, Duan Yan, Chen Yan-lin, and Xu Zheng-hui. 2007. Diversity of ants in differents habitats in Yuanmou arid-hot valley, Yunnan. Journal of Fujian College of Forestry 27(3): 272-277.
- Li Z.h. 2006. List of Chinese Insects. Volume 4. Sun Yat-sen University Press
- Mathew R., and R. N. Tiwari. 2000. Insecta: Hymenoptera: Formicidae. Pp. 251-409 in: Director; Zoological Survey of India (ed.) 2000. Fauna of of Meghalaya. Part 7. [State Fauna Series 4.] Insecta 2000. Calcutta: Zoological Survey of India, 621 pp.
- Menozzi C. 1934. Reperti mirmecofaunistici raccolti dal Prof. L di Caporiacco nelle oasi di Cufra e in altre localita del deserto Libico. Atti della Societa dei Naturalisti e Matematici di Modena (6)13(65): 153-166
- Mohanraj P., M. Ali, and K. Veerakumari. 2010. Formicidae of the Andaman and Nicobar Islands (Indian Ocean: Bay of Bengal). Journal of Insect Science 10: Article 172
- Mohanraj, P., M. Ali and K. Veenakumari. 2010. Formicidae of the Andaman and Nicobar Islands (Indian Ocean: Bay Of Bengal). Journal of Insect Science 10:172.
- Neville P.J., D.J. O'Dowd, and A.L. Yen. 2008. Issues and implications for research on disturbed oceanic islands illustrated through an ant survey of the Cocos (Keeling Islands). Journal of Insect Conservation 12: 313-323.
- Neville, P. J., O'Dowd, D. J., and Yen, A. L. 2008. Issues and implications for research on disturbed oceanic islands illustrated through an ant survey of the Cocos (Keeling) Islands. J Insect Conserv. 12:313-323.
- Neville, P.J., D.J. O'Dowd and A.L. Yen. 2008. Issues and implications for research on disturbed oceanic islands illustrated through an ant survey of the Cocos (Keeling) Islands. Insect Conserv. 12. 313-323.
- Ngoc Anh L., K. Ogata, and S. Hosoishi. 2010. Ants of agricultural fields in Vietnam (Hymenoptera: Formicidae). Bull. Inst. Trop. Agr. Kyushu Univ. 33: 1-11.
- Pfeiffer M.; Mezger, D.; Hosoishi, S.; Bakhtiar, E. Y.; Kohout, R. J. 2011. The Formicidae of Borneo (Insecta: Hymenoptera): a preliminary species list. Asian Myrmecology 4:9-58
- Pfeiffer, M., H. Cheng Tuck, and T. Chong Lay. 2008. Exploring arboreal ant community composition and co-ccurrence patterns in plantations of oil palm Elaeis guineensis in Borneo and Peninsular Malaysia. Ecography 31(1): 21-32.
- Room P. M. 1975. Diversity and organization of the ground foraging ant faunas of forest, grassland and tree crops in Papua Nez Guinea. Aust. J. Zool. 23: 71-89.
- Room, P.M. 1975. Relative Distributions of Ant Species in Cocoa Plantations in Papua New Guinea Relative Distributions of Ant Species in Cocoa Plantations in Papua New Guinea. Journal of Applied Ecology 12(1):47-61
- Stitz H. 1912. Ameisen aus Ceram und Neu-Guinea. Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin 1912: 498-514.
- Taylor R. W. 1987. A checklist of the ants of Australia, New Caledonia and New Zealand (Hymenoptera: Formicidae). CSIRO (Commonwealth Scientific and Industrial Research Organization) Division of Entomology Report 41: 1-92.
- Terayama M. 2009. A synopsis of the family Formicidae of Taiwan (Insecta: Hymenoptera). Research Bulletin of Kanto Gakuen University. Liberal Arts 17:81-266.
- Terayama Mamoru. 2009. A synopsis of the family Formicidae of Taiwan (Insecta, Hymenoptera). The Research Bulletin of Kanto Gakuen University 17: 81-266.
- Terayama, M. 2009. A synopsis of the family Formicidae of Taiwan (Insecta; Hymenoptera). The Research Bulletin of Kanto Gakuen University 17: 81-266.
- Terayama. M. and Inoue. N. 1988. Ants collected by the members of the Soil Zoological Expedition to Taiwan. ARI Reports of the Myrmecologists Society (Japan) 18: 25-28
- Tiwari R. N., B. G. Kundu, S. Roy Chowdhury, and S. N. Ghosh. 2003. Insecta: Hymenoptera: Formicidae. Fauna of Sikkim. Part 4. State Fauna Series. 9.Zool.Surv.India. i-iii, 1-512. Chapter pagination: 467-506.
- Tiwari R.N., B.G. Kundu, S. Roychowdhury, S.N. Ghosh. 1999. Insecta: Hymenoptera: Formicidae. Pp. 211-294 in: Director; Zoological Survey of India (ed.) 1999. Fauna of West Bengal. Part 8. Insecta (Trichoptera, Thysanoptera, Neuroptera, Hymenoptera and Anoplura). Calcutta: Zoological Survey of India, iv + 442 pp.
- Viehmeyer H. 1912. Ameisen aus Deutsch Neuguinea gesammelt von Dr. O. Schlaginhaufen. Nebst einem Verzeichnisse der papuanischen Arten. Abhandlungen und Berichte des Königlichen Zoologischen und Anthropologische-Ethnographischen Museums zu Dresden 14: 1-26.
- Wang W. R., S. Q. Zhang, and Z. H. Xu. 2012. A faunistic and taxonomic study of ants (Hymenoptera: Formicidae) in Shenzhen Municipality. Journal of Southwest Forestry University 32(1): 64-73.
- Wang W., S. Zhang, and Z Xu. 2012. Distribution patters of ant species in Shenzhen City. Journal of Southwest Forestry University 32(3): 70-74.
- Wang W., S. Zhang, and Z. Xu. 2012. Distribution Patterns of Ant Species in Shenzhen City. Journal of Southwest Forestry University 32(3): 69-74.
- Wetterer J. K. 2010b. Worldwide spread of the woly ant, Tetramorium lanuginosum (Hymenoptera: Formicidae). Myrmecological News 13: 81-88.
- Wheeler W. M. 1909. Ants of Formosa and the Philippines. Bulletin of the American Museum of Natural History 26: 333-345.
- Wheeler W. M. 1916. An Indian ant introduced into the United States. Journal of Economic Entomology 9: 566-569.
- Wheeler W. M. 1919. The ants of Borneo. Bulletin of the Museum of Comparative Zoology 63:43-147.
- Wheeler W. M. 1922. Ants of the American Museum Congo expedition. A contribution to the myrmecology of Africa. VIII. A synonymic list of the ants of the Ethiopian region. Bulletin of the American Museum of Natural History 45: 711-1004
- Wheeler W. M. 1927. Ants collected by Professor F. Silvestri in Indochina. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 20: 83-106.
- Wheeler W. M. 1928. Ants collected by Professor F. Silvestri in China. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 22: 3-38.
- Wheeler W. M. 1929. Ants collected by Professor F. Silvestri in Formosa, the Malay Peninsula and the Philippines. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 24: 27-64.
- Wheeler W. M. 1929. Some ants from China and Manchuria. American Museum Novitates 361: 1-11.
- Wheeler W. M. 1930. A list of the known Chinese ants. Peking Natural History Bulletin 5: 53-81.
- Wheeler W. M. 1937. Additions to the ant-fauna of Krakatau and Verlaten Island. Treubia 16: 21-24.
- Wheeler W.M. 1935. Check list of the ants of Oceania. Occasional Papers of the Bernice Pauahi Bishop Museum 11(11):1-56.
- Wheeler, William Morton. 1924. Ants of Krakatau and Other Islands in the Sunda Strait. Treubia. 5(1-3):1-20.
- Wheeler, William Morton.1935.Checklist of the Ants of Oceania.Occasional Papers 11(11): 3-56
- Wilson E.O., and G.L. Hunt. 1967. Ant fauna of Futuna and Wallis islands, stepping stones to Polynesia. Pacific Insects 9(4): 563-584.
- Yamane S. 2013. A Review of the ant fauna of the Krakatau Islands, Indonesia. Bull. Kitakyushu Mus. Nat. Hist. Hum. Hist. Ser: A, 11: 1-66
- Yamane S.; Bui T. V.; Ogata K.; Okido H.; Eguchi K. 2002. Ant fauna of Cuc Phuong National Park, North Vietnam (Hymenoptera: Formicidae). Bulletin of the Institute of Tropical Agriculture Kyushu University 25: 51-62.
- Yamane Sk. 2005. Krakatau in 1982, and the commencement of myrmecological research. The nature and Insects (Konchu to shizen) 40: 27-33.
- Zhang R. J., L. W. Liang, and S. Y. Zhou. 2014. An analysis on the ant fauna of Nonggang Nature Reserve in Guangxi, China. Journal of Guangxi Normal university: Natural Science Edition 32(3): 86-93.
- Zhang W., G. Liu, P. Zhong, and S. Zhang. 2014. Investigation of Formicidae in Luofushan Mountain. Journal of Huizhou University 34(3): 46-50.
- Zhou S.-Y. 2001. Ants of Guangxi. Guangxi Normal University Press, Guilin, China, Guilin, China. 255 pp.
- Zryanin V. A. 2011. An eco-faunistic review of ants (Hymenoptera: Formicidae). In: Structure and functions of soil communities of a monsoon tropical forest (Cat Tien National Park, southern Vietnam) / A.V. Tiunov (Editor). – M.: KMK Scientific Press. 2011. 277 р.101-124.