Myrmecia croslandi
| Myrmecia croslandi | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmeciinae |
| Tribe: | Myrmeciini |
| Genus: | Myrmecia |
| Species group: | pilosula |
| Species: | M. croslandi |
| Binomial name | |
| Myrmecia croslandi Taylor, 1991 | |
Individuals may have a single pair of chromosomes. Myrmecia croslandi (along with the locally less frequent M. impaternata) is common in Canberra parks, gardens, suburban grass lawn roadside “nature strips” and in grassy bushland. Nests of the two species are sometimes found only meters apart. Croslandi was found similarly common at localities near Armidale, NSW in Dec/Jan. 1995–96 and Nov. 1999 by JACP collectors. It is also sympatric there with M. impaternata. Several records confirm the presence of this species in SE Queensland.
Identification
Keys including this Species
Distribution
Myrmecia croslandi was described initially from the ACT, nearby NSW and Warrandyte South, VIC. It is now known also from the New England Tablelands in northeastern NSW and upland localities on the Darling Downs of SE QLD, from Glen Innes in northeastern NSW, and from near Cobangra, VIC.
Latitudinal Distribution Pattern
Latitudinal Range: -27.11° to -37.6°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Australasian Region: Australia (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
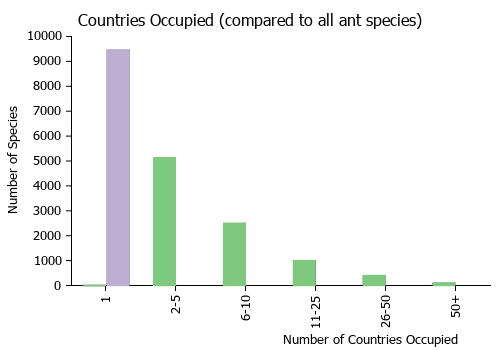
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
Sympatric variously with Myrmecia impaternata and Eastern Myrmecia pilosula, and with Myrmecia haskinsorum (and Eastern pilosula) at Corang River Bridge (-35 12, 150 03). Most known records of M. impaternata were taken in sympatry with M. croslandi.
Morphology
Myrmecia use their large eyes to locate prey and to find their way back to the nest from their foraging forays. Ogawa et al. (2015) wanted to know how complex the color reception in ants may be and they felt M. croslandi was a good candidate for exploring this question. They provided evidence about photoreceptors in M. croslandi and Myrmecia vindex that show ants can have sophisticated trichromatic color reception. Their abstract (Ogawa et al. 2015): Ants are thought to be special among Hymenopterans in having only dichromatic colour vision based on two spectrally distinct photoreceptors. Many ants are highly visual animals, however, and use vision extensively for navigation. We show here that two congeneric day- and night-active Australian ants have three spectrally distinct photoreceptor types, potentially supporting trichromatic colour vision. Electroretinogram recordings show the presence of three spectral sensitivities with peaks (lmax) at 370, 450 and 550 nm in the night-active Myrmecia vindex and peaks at 370, 470 and 510 nm in the day-active Myrmecia croslandi. Intracellular electrophysiology on individual photoreceptors confirmed that the night-active M. vindex has three spectral sensitivities with peaks (lmax) at 370, 430 and 550 nm. A large number of the intracellular recordings in the night-active M. vindex show unusually broad-band spectral sensitivities, suggesting that photoreceptors may be coupled. Spectral measurements at different temporal frequencies revealed that the ultraviolet receptors are comparatively slow. We discuss the adaptive significance and the probability of trichromacy in Myrmecia ants in the context of dim light vision and visual navigation.
Determination Clarifications
It was discussed as “M. pilosula” by Crosland and Crozier (1986), and as “M. (pilosula) n=1” by Imai & Taylor (1989).
Castes
Phylogeny
| Myrmecia |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Based on Mera-Rodríguez et al. (2023).
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- croslandi. Myrmecia croslandi Taylor, 1991c: 288 (w.k.) AUSTRALIA (New South Wales, Australian Capital Territory, Victoria).
- Type-material: holotype worker, 79 paratype workers.
- Type-locality: holotype Australia: New South Wales, immediately NE Corang River Bridge (35°08’S, 150°02’E), on Braidwood-Nowra Road, nr Charleyong, xii.1989, HI89-031 (H.T. Imai, et al.); paratypes: 15 workers with same data, 14 workers with same data but HI89-030, 8 workers with same data but HI89-032, 6 workers with same data but xii.1987, HI87-136, 6 workers with same data but xii.1987, HI87-148, 6 workers with same data but xii.1987, HI87-150, 6 workers with same data but xii.1987, HI87-151, 6 workers with same data but xii.1987, HI87-153, 6 workers with same data but xii.1987, HI87-154, 6 workers with same data but xii.1987, HI87-157.
- [Note: Taylor, 2015a: 503, gives slightly different type-locality data: “immediately E to NE Corang River Bridge (-35 12, 150 03) on Nerriga Road, nr Braidwood”.]
- Type-depository: ANIC.
- Imai, Taylor & Crozier, 1994: 145 (k.).
- Status as species: Bolton, 1995b: 271; Taylor, 2015a: 503.
- Distribution: Australia.
Type Material
- Holotype, worker, immediately NE of Corang River Bridge on Braidwood-Nowra Road , near Charleyong, New South Wales, Australia, Australian National Insect Collection.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Taylor (2015) - General features as illustrated and in key couplets 1, 2, 5 & 6. Distinguished from other pilosula-complex species by its robust form, more massive petiolar node, especially versus representatives of the two races of Myrmecia pilosula (compare Figures) and other details, as specified in the key. Middle and hind tibiae medium brown, matching the femora, the tibial apices minutely lightly infuscated at the bases of the reddish-orange spurs. Larger workers of both races of M. pilosula often closely resemble those of Myrmecia croslandi. Western M. pilosula is then distinguishable by its reddish-orange hind tibiae (see below under that species), but Eastern M. pilosula and M. croslandi are essentially identical in leg coloration (see key couplet 6 for their discrimination).
The holotype and smallest and largest available specimens have the following dimensions (mm): TL = 13.54, 12.46, 13.53; HW = 2.63, 2.54, 2.79; HL = 2.35, 2.37, 2.51; CI = 112, 107, 111; EL =1.02, 1.02, 1.08; OI = 39, 40, 39; SL = 2.02, 1.99, 2.06; SI = 77, 78, 74; PW = 1.70, 1.60, 1.81; WL = 3.88, 3.73, 4.06; PetW = 1.06, 0.93, 1.14; PpetW = 1.59, 1.43, 1.69.
Karyotype
- n = 2, 2n = 3, karyotype = 1M+1M+1A (Australia) (Taylor, 1991; Meyne et al., 1995; Hirai et al., 1994; Hirai et al., 1996; Imai et al., 1992; Imai et al., 1994) (Complex pilosula).
- n = 2, 2n = 3, karyotype = 1M+1SM +1M (Australia) (Taylor, 1991; Meyne et al., 1995; Hirai et al., 1994; Hirai et al., 1996; Imai et al., 1992; Imai et al., 1994) (Complex pilosula).
- n = 2, 2n = 4, karyotype = 2SM +1A+1A (Australia) (Taylor, 1991; Meyne et al., 1995; Hirai et al., 1996; Imai et al., 1992; Imai et al., 1994) (Complex pilosula).
- n = 1, 2n = 2 (Australia) (Taylor, 1991; Meyne et al., 1995; Hirai et al., 1994; Hirai et al., 1996; Imai et al., 1994) (Complex pilosula).
Taylor (2015) - Workers and queens in some colonies have the minimum possible eukaryote chromosome count of 2N=2. Myrmecia croslandi is widely celebrated as the only animal other than the nematode Diploscapter coronata known to possess a single pair of chromosomes. Imai & Taylor (1989) reported that its chromosome numbers in fact vary, ranging 2n=2, 3 or 4, and that croslandi demonstrates highly complicated chromosome polymorphisms, including telomere fusion, shift of centromeric activity by centromeric inactivation, salutatory growth of constitutive heterochromatin (C+), and AM inversion. Typical croslandi karyotypes with 2n=2 (2K=2Mci), and 2n=3 (2K=lAc+1M+1Mci) were illustrated by Imai, Taylor et al. (1994, figs 5a, 5b), and karyological details discussed by Imai, Hirae et al. (1992).
Etymology
Named for Michael W. J. Crosland, who as a student of R. H. Crozier at the University of New South Wales, Sydney, discovered the 2n=2 chromosome count while experimenting with the Crozier/Imai air-drying technique of chromosome preparation for microscopy (to great initial consternation that the technique had failed, but later celebration). Crosland had collected the subject specimens shortly before at Tidbinbilla Nature Reserve near Canberra.
References
- Barros, L.A.C., Aguiar, H.J.A.C., Teixeira, G.C., Souza, D.J., Delabie, J.H.C., Mariano, C.S.F. 2021. Cytogenetic studies on the social parasite Acromyrmex ameliae (Formicidae: Myrmicinae: Attini) and its hosts reveal chromosome fusion in Acromyrmex. Zoologischer Anzeiger 293, 273–281 (doi:10.1016/j.jcz.2021.06.012).
- Borowiec, M.L., Moreau, C.S., Rabeling, C. 2020. Ants: Phylogeny and Classification. In: C. Starr (ed.), Encyclopedia of Social Insects (doi:10.1007/978-3-319-90306-4_155-1).
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Cardoso, D. C., Cristiano, M. P. 2021. Karyotype diversity, mode, and tempo of the chromosomal evolution of Attina (Formicidae: Myrmicinae: Attini): Is there an upper limit to chromosome number? Insects 1212, 1084 (doi:10.3390/insects12121084).
- Greiner, B., Narendra, A., Reid, S.F., Dacke, M., Ribi, W.A., Zeil, J. 2007. Eye structure correlates with distinct foraging-bout timing in primitive ants. Current Biology 17(20): R879-880.
- Imai, H. T.; Taylor, R. W.; Crozier, R. H. 1994. Experimental bases for the minimum interaction theory. I. Chromosome evolution in ants of the Myrmecia pilosula species complex (Hymenoptera: Formicidae: Myrmeciinae). Jpn. J. Genet. 69:137-182 (page 145, karyotype described)
- Jayatilaka, P., Raderschall, C.A., Narendra, A. & Zeil, J. 2013. Individual foraging patterns of the jack jumper ant Myrmecia croslandi (Hymenoptera: Formicidae). Myrmecological News 19, 75-83.
- Jayatilaka,P., Narendra,A., Reid,S.F., Cooper,P., Zeil,J. 2011. Different effects of temperature on foraging activity schedules in sympatric Myrmecia ants. The Journal of Experimental Biology. 214:2730-2738. doi:10.1242/jeb.053710.
- Kamhi, J.F., Barron, A.B., Narendra, A. 2020. Vertical lobes of the Mushroom Bodies are essential for view-based navigation in Australian Myrmecia ants. Current Biology 30, 1–6 (doi:10.1016/J.CUB.2020.06.030).
- Lorite, P., Palomeque, T. 2010. Karyotype evolution in ants (Hymenoptera: Formicidae), with a review of the known ant chromosome numbers. Myrmecological News 13: 89-102.
- Mera-Rodríguez, D., Jourdan, H., Ward, P.S., Shattuck, S., Cover, S.P., Wilson, E.O., Rabeling, C. 2023. Biogeography and evolution of social parasitism in Australian Myrmecia bulldog ants revealed by phylogenomics. Molecular Phylogenetics and Evolution 186, 107825 (doi:10.1016/j.ympev.2023.107825).
- Narendra, A., Alkaladi, A., Raderschall, C.A., Robson, S.K.A., Ribi, W.A. 2013. Compound eye adaptations for diurnal and nocturnal lifestyle in the intertidal ant, Polyrhachis sokolova. PLoS ONE 8, e76015 (doi:10.1371/journal.pone.0076015).
- Narendra, A., Gourmaud, S. & Zeil, J. 2013. Mapping the navigational knowledge of individually foraging ants, Myrmecia croslandi. Proceedings of the Royal Society B. 280:20130683 doi:10.1098/rspb.2013.0683
- Narendra, A., Ramirez-Esquivel, F., Ribi, W.A. 2016. Compound eye and ocellar structure for walking and flying modes of locomotion in the Australian ant, Camponotus consobrinus. Scientific Reports 6, 22331 (doi:10.1038/srep22331).
- Narendra, A., Reid, S.F., Greiner, B., Peters, R.A., Hemmi, J.M., Ribi, W.A., Zeil, J. 2010. Caste-specific visual adaptations to distinct daily activity schedules in Australian Myrmecia ants. Proceedings of the Royal Society B: Biological Sciences 278, 1141–1149 (doi:10.1098/rspb.2010.1378).
- Narendra, A., Reid, S.F., Greiner, B., Peters, R.A., Hemmi, J.M., Ribi, W.A., Zeil, J. 2011. Caste-specific visual adaptations to distinct daily activity schedules in Australian Myrmecia ants. Proc. R. Soc. B. 278:1141-1149 doi:10.1098/rspb.2010.1378.
- Ogawa, Y., M. Falkowski, A. Narendra, J. Zeil, and J. M. Hemmi. 2015. Three spectrally distinct photoreceptors in diurnal and nocturnal Australian ants. Proceedings of the Royal Society of London B: Biological Sciences. 282:20150673. doi:10.1098/rspb.2015.0673
- Raderschall, C.A., Narendra, A., Zeil, J. 2016. Head roll stabilisation in the nocturnal bull ant Myrmecia pyriformis: implications for visual navigation. The Journal of Experimental Biology 219, 1449–1457 (doi:10.1242/jeb.134049).
- Reznikova, Z. 2020. Spatial cognition in the context of foraging styles and information transfer in ants. Animal Cognition. (doi:10.1007/s10071-020-01423-x).
- Taylor, R. W. 1991c. Myrmecia croslandi sp. n., a karyologically remarkable new Australian jack-jumper ant (Hymenoptera: Formicidae: Myrmeciinae). J. Aust. Entomol. Soc. 30:288 (page 288, worker, karyotype described)
- Taylor, R.W. 2015. Ants with Attitude: Australian Jack-jumpers of the Myrmecia pilosula species complex, with descriptions of four new species (Hymenoptera: Formicidae: Myrmeciinae). Zootaxa. 3911:493–520. doi:10.11646/zootaxa.3911.4.2
- Taylor, R.W., Imai, H.T., Hasegawa, E., Beaton, C.D. 2018. A unique conjunction: Evidence for gynogenesis accompanying haplodiploid sex determination in the Australian ant Myrmecia impaternata Taylor. Psyche: A Journal of Entomology 2018, 1–7 (doi:10.1155/2018/2832690).
- van der Kooi, C.J., Stavenga, D.G., Arikawa, K., Belušič, G., Kelber, A. 2020. Evolution of insect color vision: From spectral sensitivity to visual ecology. Annual Review of Entomology 66, annurev-ento-061720–071644. (doi:10.1146/annurev-ento-061720-071644).
References based on Global Ant Biodiversity Informatics
- Imai, H. T.; Taylor, R. W.; Crozier, R. H. 1994. Experimental bases for the minimum interaction theory. I. Chromosome evolution in ants of the Myrmecia pilosula species complex (Hymenoptera: Formicidae: Myrmeciinae). Japanese Journal of Genetics 69:174. [1994-04-25] PDF 126063
- Jayatilaka P., C. A. Raderschall, A. Narendra, and J. Zeil. 2013. Individual foraging patterns of the jack jumper ant Myrmecia croslandi (Hymenoptera: Formicidae). Myrmecological News 19: 75-83.
- Taylor R. W. 2015. Ants with Attitude: Australian Jack-jumpers of the Myrmecia pilosulaspecies complex, with descriptions of four new species (Hymenoptera: Formicidae: Myrmeciinae). Zootaxa 3911(4): 493-520.